Abstract
| Table of Contents | |
|---|---|
| Summary | 119 |
| 1. Introduction | 119 |
| 1.1 The gastrointestinal ecosystem | 119 |
| 1.2 Groups of intestinal parasitic worms that infect humans | 121 |
| 1.2.1 Flukes or trematodes | 121 |
| 1.2.2 Tapeworms or cestodes | 122 |
| 1.2.3 Roundworms or nematodes | 123 |
| 1.3 How worms may affect human nutrition and growth | 126 |
| 1.4 Design of studies estimating the impact of worms | 128 |
| 1.5 Aims | 128 |
| 2. Factors affecting the impact of intestinal worms | 128 |
| 2.1 Species of intestinal worm | 128 |
| 2.2 Prevalence of infection | 129 |
| 2.3 Number and distribution of worms | 132 |
| 2.4 Duration of infection | 135 |
| 2.5 Rate of reinfection | 135 |
| 2.6 Summary | 136 |
| 3. Factors affecting the impact of treatment | 137 |
| 3.1 Study design: controls and randomization | 138 |
| 3.2 Anthelmintic drugs | 138 |
| 3.3 Intervals between treatments | 141 |
| 3.4 Duration of follow‐up | 142 |
| 3.5 Outcomes measured and the need for controls | 142 |
| 3.6 Initial nutritional status | 143 |
| 3.7 Age of subjects | 144 |
| 3.8 Remedial therapy after treatment | 144 |
| 3.9 Summary | 145 |
| 4. Aims and methods of the meta‐analysis | 145 |
| 4.1 Search terms | 145 |
| 4.2 Inclusion criteria | 146 |
| 4.3 Exclusion criteria | 147 |
| 4.4 Meta‐analysis | 147 |
| 5. Results of the meta‐analysis | 147 |
| 5.1 Geographic origin of studies | 150 |
| 5.2 Estimates of effects | 150 |
| 5.3 The figures and how to interpret them | 150 |
| 5.4 Sources of error or bias | 150 |
| 6. Discussion | 153 |
| 6.1 Magnitude of effects | 153 |
| 6.2 Treatment alone is not enough | 159 |
| 6.3 The Cochrane Collaboration Review | 161 |
| 6.4 Characteristics of an ideal study | 162 |
| 6.5 Implications for programmes | 163 |
| 6.6 Conclusions | 166 |
| Acknowledgements | 167 |
| References | 167 |
| Appendix: Summary of papers identified for the review | 177 |
Summary
More than a half of the world's population are infected with one or more species of intestinal worms of which the nematodes Ascaris lumbricoides, Trichuris trichiura and the hookworms are the most common and important in terms of child health. This paper: (1) introduces the main species of intestinal worms with particular attention to intestinal nematodes; (2) examines how such worms may affect child growth and nutrition; (3) reviews the biological and epidemiological factors that influence the effects that worms can have on the growth and nutrition of children; (4) considers the many factors that can affect the impact of treatment with anthelmintic drugs; (5) presents the results of a meta‐analysis of studies of the effect of treating worm infections on child growth and nutrition; (6) discusses the results in terms of what is reasonable to expect that deworming alone can achieve; (7) describes some important characteristics of an ideal study of the effects of deworming; and (8) comments on the implications for programmes of recommendations concerning mass deworming.
Keywords: intestinal worms, anthelmintics, children, growth, nutrition
Key messages
-
•
The effects of intestinal worms depend on the species, the mixture of species, the duration of infection and the number of worms.
-
•
The distribution of worms among hosts is highly skewed so that only a minority of infected individuals have moderate to heavy infections and are likely to be diseased.
-
•
The impact of infections will also depend on the size and nutritional status of the host.
-
•
Treating worms can lead to improvements in growth and nutritional status but deworming alone does not treat any underlying nutritional deficits that have been caused or made worse by worms, so extra energy, protein and micronutrients are required.
1. Introduction
Parasitic worms are among the most common and widespread infections of humans in the world today. Using recent estimates of the prevalence of the four main species of intestinal nematode worm (de Silva et al. 2003) a simple calculation that assumes the probability of infection with one worm is independent of infection with another, indicates that about 48% of the 5 billion or so people living in the developing world are infected with at least one species, while almost 10% are infected with at least two species. But, if some 2.3 billion people in the developing world are infected with intestinal nematode worms, why is not disease due to worms more common, and why do not they seem to have a greater and more noticeable impact on the health of children? This review will attempt to explain why. It will review the studies that have been performed to examine the impact of treating intestinal nematode worms on children's nutritional status and growth, and it will examine the scientific and experimental problems with estimating the impact that worms in general have on human health.
1.1 The gastrointestinal ecosystem
The human intestinal tract provides a protected habitat for several hundred species of viruses, bacte ria, yeasts, protozoa and worms. All the organisms that live in the intestinal ecosystem are parasites, because they are dependent for their existence on their host, and the basis of this dependence is usually nutritional (Hall 1985). The parasitic lifestyle is highly successful for worms in general mainly because, once established within a host, there are no predators and life is a sheltered steady state with a constant supply of nutrients that is sustained by the host's homeostatic mechanisms.
Because the gut is a cavity within the host, it is said to be immunologically privileged, as the organisms living there are not exposed to the full force of the human immune system. Nevertheless, intestinal worms do elicit an immune response, and hookworms and whipworms in particular come into contact with both the cellular and humoral immune systems to elicit a Th‐2 responses and cause a rise in the concentration of immunoglobulin E (Else 2005). But the fact that intestinal worms persist and are not expelled from the gut indicates that they are able to evade these immune responses, although the mechanisms by which they achieve this are unclear.
The only important non‐specific barrier to infection is hydrochloric acid, secreted as ions into the stomach lumen by the parietal cells in the gut wall. Although this acid can kill infectious stages of many potential pathogens, paradoxically it is also a necessary stimulus for the establishment of many parasites: exposure to acid is required for the excystment of Giardia duodenalis (Hautus et al. 1988; al‐Tukhi et al. 1991) and may be a necessary stimulus for the eggs of Ascaris lumbricoides to hatch, along with warmth and exposure to bile salts. Once in the intestine, the site where the worms come to maturity, all parasites of the gut have a body surface that is resistant to the action of the host's digestive enzymes, while some worms have developed specific antienzymes (Uglem & Just 1983), presumably for self‐protection. The role of antienzymes in causing malnutrition is putative rather than proven, and it seems most likely that they act locally to prevent damage to the worms’ surface by host enzymes, rather than being secreted to have a widespread effect in the gut and thus perhaps on human nutrition.
The infectious stages of parasites have an easy way to enter their host, usually through the mouth as a contaminant of food, water or fingers, while the next generation leaves the body in faeces through the anus in the form of spores, cysts, eggs or larvae. There are exceptions: a few parasites of the gut enter the body through the skin, notably the larvae of hookworms and Strongyloides stercoralis.
The major problem for parasitic worms is to get from one host to another, a journey that is facilitated in several different ways:
-
•
by producing large numbers of infectious stages to increase the chances of infecting a new host; a fertilized female A. lumbricoides, for example, may produce up to 200 000 eggs a day (Sinniah 1982), therefore millions in a lifetime;
-
•
by producing resistant infectious stages that can withstand adverse conditions; the eggs of species of Ascaris can survive for several months or years in warm, humid and sheltered conditions (Gaasenbeek & Borgsteede 1998), and are resistant even to 10% formalin (Sandars 1951) though not to exposure to ultra‐violet light or to desiccation (Crompton 1989);
-
•
by infecting an intermediate host in which the parasite both multiplies and is dispersed, a feature of the life cycle of many trematodes, a group of flatworms whose species often reproduce in snails from which larval stages are released that are infectious to humans;
-
•
by infecting or encysting on foods that are consumed by a new host (Fried et al. 2004);
-
•
by the behaviour of infected people that puts others at risk of infection, such as defecating in the open, so that infectious stages are spread in the environment (Kilama 1989); and
-
•
by taking advantage of the behaviours that put people at risk of infection, such as pica (Geissler et al. 1998); by using fresh human faeces (sometimes called ‘nightsoil’) as a fertilizer (Pan et al. 1954; Needham et al. 1998); and by poor personal hygiene.
As the gastrointestinal ecosystem offers such a rich habitat, it has been colonized by an enormous number of different species. The next section introduces the major species of worms that live in the human gastrointestinal tract.
1.2 Groups of intestinal parasitic worms that infect humans
The variety of general and specific names given to worms can be quite confusing to a novice (see Box 1). The terms ‘helminths’ and ‘worms’ are generic names for metazoan (multicellular) parasites that are classified by helminthologists into two main Phyla:
Box 1. How worms are named
Worms go under a variety of general and specific names derived from different languages, but mostly old English, Latin or Greek. ‘Worm’ is derived from the old English word wyrm, meaning a snake or a dragon. ‘Worm’ is also associated with the Latin vermis from which comes the English words vermicide and vermifuge, a drug for treating worms. The generic term ‘helminths’ is an English word derived from the Greek word for worms, helmins. From this root is derived the term anthelmintic (sometimes anthelminthic or antihelminthic), a drug to treat worms. The name of the phylum Platyhelminthes combines the Greek terms platys, meaning broad or flat, and the word for worm. The Platyhelminths include two groups: the tapeworms (Old English ‘tape’ meaning tape, combined with ‘worm’) or cestodes (derived from the Greek word kestos meaning a strap); and the flukes (Old English term derived from the name for a type of fish called a plaice or flounder, which the worms look like) also called trematodes (derived from the Greek word trema meaning orifice or hole and eidos, meaning ‘in form’). The Phylum Nematoda (derived from the Greek nema, a thread, and eidos, meaning ‘in form’) are classified as helminths (but not Platyhelminths) as they are roundworms (derived from old French rond meaning round), not flat worms. Each species has a name in Latin that is a noun (which takes an upper case first letter) followed by an adjective (which takes a lower case first letter). For example the Latin name Ascaris lumbricoides is derived from the Greek word Askaris, meaning intestinal worm, and the Latin word lumbricus, meaning worm‐like. The worm was given its name in 1758 by Carolus Linnæus, the father of nomenclature, who apparently had not heard of tautology.
-
•
Nematoda: the nematodes or roundworms, such as A. lumbricoides and Trichuris trichiura; and
-
•
Platyhelminthes: the flatworms, which contain two important Classes of parasites of humans:
-
∘
Trematoda: the flukes, such as Fasciolopsis buski and Metagonimus yokogawa;
-
∘
Cestoidea, subclass Eucestoda: the tapeworms, such as Taenia saginata and Diphyllobothrium latum.
-
∘
Over 340 species of helminths have been recorded in association with humans (Coombs & Crompton 1991) but most are rare zoonoses – infections of animals that can be contracted by humans. Table 1 lists the names of the most common species of helminths that live in the human intestine. The following section describes the life cycles of the most common species of intestinal flukes, tapeworms and roundworms.
Table 1.
The names of the most common intestinal helminth infections of humans, their infectious stages, the obligatory intermediate host and the stage that is infectious to humans
| Latin name | English name | Class or Phylum | Infectious stage in faeces | Intermediate host | Stage infectious to humans |
|---|---|---|---|---|---|
| Fasciolopsis buski | Intestinal fluke | Trematoda | Egg | Water snail | Metacercaria on vegetation |
| Echinostoma spp. | Intestinal fluke | Trematoda | Egg | Water snail | Metacercaria in snails |
| Taenia * saginata | Beef tapeworm | Cestoidea | Egg in proglottid | Cow and bovids | Cysticercus in beef |
| Taenia * solium | Pork tapeworm | Cestoidea | Egg in proglottid | Pig and swine | Cysticercus in pork |
| Hymenolepis nana | Dwarf tapeworm | Cestoidea | Egg in proglottid | Beetle, flea | Cysticercoid in insect |
| Hymenolepis diminuta | Rat tapeworm | Cestoidea | Egg in proglottid | Insect | Cysticercoid in insect |
| Diphyllobothrium latum | Fish tapeworm | Cestoidea | Egg | Fish | Plerocercoid in raw fish |
| Enterobius vermucularis | Pinworm | Nematoda | Egg | No | Larva in egg shell |
| Strongyloides stercoralis | Threadworm | Nematoda | Egg or larva | No | Free‐living larva |
| Ascaris lumbricoides | Large roundworm | Nematoda | Egg | No | Larva in egg shell |
| Trichuris trichiura | Whipworm | Nematoda | Egg | No | Larva in egg shell |
| Ancylostoma duodenale | Hookworm | Nematoda | Egg | No | Free‐living larva |
| Necator americanus | Hookworm | Nematoda | Egg | No | Free‐living larva |
Also known as genus Taeniarhynchus.
1.2.1 Flukes or trematodes
The two main species of intestinal trematode that infect humans listed in Table 1 are not widespread, although F. buski occurs focally in south‐east Asian countries such as Thailand and the Philippines (Waikagul 1991) and in the Indian subcontinent (Gilman et al. 1982; Chandra 1984) Fasciolopsis buski is a zoonosis, and usually infects dogs and pigs, two animals closely associated with humans (Mas‐Coma et al. 2005). A study in China reported an association between malnutrition and infection with flukes including F. buski, but the prevalence of this species was relatively low and the main pathogenic species was judged to be Schistosoma japonicum (Zhou et al. 2005).
Some 16 species of Echinostoma have been reported to infect humans (Huffman & Fried 1990; Carney 1991), which makes it the most common genus of intestinal fluke, but another seven species of gut flukes from a variety of Trematode families have been recorded including the Fasciolidae, Heterophyidae, Lecithodriidae, Microphallidae, Paramphistomatidae and Plagiorchiidae (Waikagul 1991). They are all zoonoses: infections of humans occurs by eating freshwater fish or shellfish, and the normal hosts are fish‐eating animals such as cats and birds. Infections in humans are mainly found in adults in Asia who eat undercooked intermediate hosts such as crabs, frogs or fish, or in children who swallow metacercariae that have encysted on vegetation, such as water caltrop.
Infections with intestinal flukes are not common, even among adults, and are rarer still among children, so there is no known association with malnutrition.
The members of the order Schistosoma are not included in this review because they are not parasites of the intestinal lumen: they live in the portal blood vessels around the gut (S. mansoni and S. japonicum) or urinary bladder (S. haematobium).
1.2.2 Tapeworms or cestodes
Of the most common tapeworms of humans, the three species of Taenia tend not to be found among children as they are transmitted by eating undercooked beef (T. saginata) or pork (T. solium = Taeniarhynchus solium and Taenia asiatica) (Eom & Rim 1993; Macpherson 2005). These foods are not commonly eaten by poor children, or are proscribed in some parts of the world. The adult worms live in the small intestine and release their eggs in packets called proglottides, a living section that breaks off the posterior end of a growing worm (Pawlowski & Schultz 1972). The proglottides of T. saginata are motile and can crawl away from a human stool deposited on the ground (see Fig. 1), whereas the proglottides of T. solium do not show this activity. This behaviour occurs because cattle do not eat human faeces, but pigs do. When the eggs of Taenia species are swallowed by a suid or bovid species, they hatch, penetrate tissues and develop in muscles or organs to become infective cysticercoids. Humans become infected by eating raw or undercooked beef or pork. Infections can be common among people who habitually eat undercooked meat, such as ethnic groups that live in the Rift Valley of East Africa (Hall et al. 1981) and, because the eggs are passed in packets rather than loose in the faeces, infections can be missed during the microscopical examination of faecal samples (Hall et al. 1981).
Figure 1.
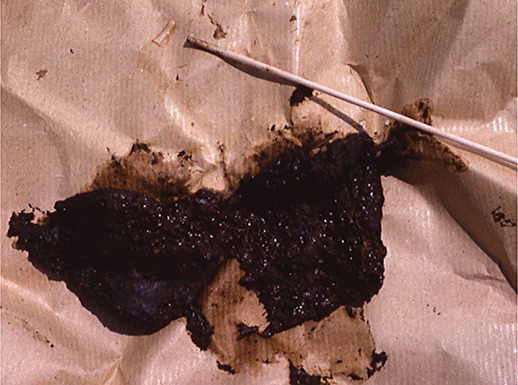
The proglottid of Taenia saginata (at the end of the stick) crawling away from a human stool in a trail of mucus. The sample was collected from a Pokot man in western Kenya, an ethnic group that traditionally enjoys eating undercooked beef (Hall et al. 1981).
The main concern for disease in humans is the possibility that the eggs of T. solium may hatch in the human intestine and develop in tissues to cause cysticercosis. If this happens in the brain it can lead to epilepsy (Newell et al. 1997). When pigs infected with T. solium were given to appease guerrillas fighting the government in west New Guinea, there was an outbreak of cysticercosis. This came to attention when people with severe burns appeared at hospitals: they had experienced an epileptic fit when sleeping next to a fire for warmth at night and had fallen into the flames (Gajdusek 1978).
The effect of tapeworms in the intestine is minimal, probably because their relative mass is small in comparison with their host. It is also thought that the presence of existing worms may perhaps inhibit the establishment of additional worms, although this is hard to prove without deliberately infecting people. There is no known association between infections with Taenia spp. and malnutrition in children.
Hymenolepis nana is a widespread parasite of children, but the reported prevalence rarely exceeds 20% and is usually less than 5% (Khalil et al. 1991; Mason & Patterson 1994; Sirivichayakul et al. 2000). The worm can persist by means of autoinfection, a process in which eggs hatch and mature in the human gut to form adults, without passing into the environment in the normal way to infect an insect intermediate host.
Hymenolepis diminuta is usually a parasite of rodents, but it is found in children in situations in which they come into contact with rat or human faeces containing the worms’ eggs.
Both species of Hymenolepis are associated with malnutrition, in that they tend to occur among children living in poor and unhygienic communities, but there have been no studies looking at the impact of treatment to suggest that they cause malnutrition.
Diphyllobothrium latum is a notable tapeworm because it selectively absorbs vitamin B12 from the diet of its host or may interfere with absorption, which occurs only in the last third of the ileum; this can lead to pernicious anaemia (Nyberg 1963). Infections occur by eating raw freshwater fish containing a plerocercoid larva, and were once common in Scandinavian countries such as Finland (Raisanen & Puska 1984). This species has been reported all over the world, but mostly as curious case reports. It is not a common cause of anaemia in young children mainly because fish is an expensive food, and in most communities it is not commonly eaten raw, especially by children.
1.2.3 Roundworms or nematodes
Of the six species of nematode worms listed in Table 1, Enterobius vermicularis is found worldwide but is rarely a cause of serious disease, and is more a cause of irritation. The female worms lay their eggs around the anus at night. This causes itching and pruritis that may occasionally lead to peri‐anal sepsis in young children (Mahomed et al. 2003), probably because they scratch themselves. Infections have been reported to cause enuresis (Otu‐Bassey et al. 2005) and are very rarely associated with appendicitis (Arca et al. 2004).
Enterobius vermicularis tends to be most common among very young children, especially in kindergartens or among children living in institutions, probably because their personal habits are not well developed and they are in close physical contact with other children (Song et al. 2003; Remm 2006). It is a difficult infection to diagnose efficiently because the eggs are not often seen in faeces, so it is necessary to press sticky cellophane tape over the peri‐anal skin of a child, usually after a night's sleep when the worms have laid their eggs, and examine the tape under a microscope (Celiksoz et al. 2005). The itching may affect a child's sleep, but is not known to be a cause of malnutrition or poor growth.
Infections with Strongyloides stercoralis are also associated with poor hygiene, close contact between people and a lack of sanitary facilities. Infections have been reported among children in nursery schools and among adults in psychiatric institutions (Braun et al. 1988; Gatti et al. 2000). The worm can persist by a process of autoinfection in which larvae hatch in the large intestine and burrow directly into the gut wall, so emulating a naturally acquired infection (Schad 1989).
Infections with S. stercoralis can be transmitted directly from person to person by exposure to fresh faeces in the immediate living environment. A study in Bangladesh found that infections with S. stercoralis in people living in an urban slum were associated with households that lacked a latrine and had an earthen floor that may help larvae to survive (Hall et al. 1994). But when these factors were controlled for, the aggregation of infections may have been due not only to shared risk factors, but to a genetic predisposition that could also have contributed to infection (Conway et al. 1995).
Although hyperinfections with S. stercoralis can be dangerous in immunocompromised patients (Keiser & Nutman 2004), such as those being treated with immunosupressants (Schaeffer et al. 2004) or in the elderly, little is known about how many children are infected in the world today, so the worm's status as a cause of malnutrition and poor growth is unknown.
The four main nematode worms most commonly associated with malnutrition and disease in children are A. lumbricoides, T. trichiura and both species of hookworms, Ancylostoma duodenale and Necator americanus. These worms are sometimes called soil‐transmitted helminths. As this term refers to their mode of transmission, the generic term intestinal nematodes will be used here, which infers direct consequences for human health, and is perhaps more informative.
Ascaris lumbricoides is the largest intestinal nematode worm to infect humans. An adult female A. lumbricoides typically weighs between 4 and 7 g, but can weigh up to 9 g and grow as long as 40 cm. Male worms are smaller, and weigh 2–3 g. Adult worms usually inhabit the jejunum (Crompton 1989) where they feed on intestinal contents, but worms may be found higher and lower in the gut when present in large numbers, perhaps because of competition for living space. Worms may sometimes migrate into unusual sites such as the bile or pancreatic ducts, which they can block and cause acute and life‐threatening disease (Sandouk et al. 1997; Ferreyra & Cerri 1998). Adult A. lumbricoides have a tendency to wander if irritated and worms have been extracted from the nose and Eustachian tube (Jain & Pahuja 1988; Fagan & Prescott 1993).
Adult A. lumbricoides maintain their position in the intestine by swimming against the flow of food, and when they die, they are carried out of the body in the faeces. Ascaris lumbricoides is the only intestinal nematode worm that is easily seen and identified in faeces, and is the only species of nematode for which anthelmintic treatment is visibly successful. This was the basis of a long‐running Japanese family planning programme: 1 because the expulsion of A. lumbricoides from the gut offered manifest evidence of the effectiveness of treatment it provided an entry point to households to encourage women to use family planning.
An adult female A. lumbricoides may produce up to 200 000 eggs a day (Sinniah 1982) in a life span of 12–18 months (Anderson & May 1991), but there is good evidence of both density‐dependent fecundity (Hall & Holland 2000) and geographical variation in the number of eggs produced per female worm (Hall & Holland 2000). This means that worms produce fewer eggs when there are many present in the gut, and that there is not a linear relationship between the number of worms in a host and the concentration of eggs in faeces. The consequence is that the concentration of eggs in a faecal sample from a Bangladeshi child, for example, is not necessarily equivalent in terms of worm burden to the same concentration of eggs in a sample from an Iranian child (Hall & Holland 2000). As any given concentration of eggs in faeces may not reflect the same number of worms in different parts of the world, it means that the use of fixed ranges of egg counts to classify the intensity of infection with intestinal nematode worms is scientifically dubious. This is discussed in more detail in Section 2.
Freshly excreted A. lumbricoides eggs are not immediately infectious and take 10–14 days to embryonate in the environment at 30°C, or about 50 days at 17°C (Pawlowski & Arfaa 1984). This means that old faeces are a source of infection, not fresh faeces, and the soil on which they lie may no longer bear evidence of faecal contamination. A new infection occurs when mature eggs are swallowed as a contaminant of food or fingers. When an egg comes into contact with bile acids, the larva breaks out of the egg case and burrows through the intestinal wall. After a few days migration through the blood stream to the liver and then to the lungs, the developing larvae break into the alveoli and are coughed up and swallowed. Large numbers of larvae can cause a verminous pneumonia (Gelpi & Mustafa 1968; Tomashefski et al. 1989; Valentine et al. 2001). The larvae pass through the stomach and into the small intestine for a second time, where they grow and mature to become adults. Although this migration through tissues exposes the immune system to Ascaris antigens and stimulates an immune response, it does not seem to lead to protective immunity, at least not in all individuals, although some individuals may develop partial immunity (see Section 2.4).
The adults of T. trichiura live in the large intestine and caecum (Bundy & Cooper 1989). These nematode worms insert their whip‐like anterior end into the gut wall and secrete enzymes and a specific protein that causes a syncytium to form (Drake et al. 1994), which provides an easily ingested liquid food. The penetration of the worm into tissues also causes inflammation and bleeding so that, when large numbers of worms are present, they can cause dysentery and even rectal prolapse (Bundy & Cooper 1989). Each female worm produces 3000–20 000 eggs a day (Bundy & Cooper 1989). The eggs mature in the environment to form an infectious larva within the egg shell in about 10–14 days. When a mature egg is swallowed the larva hatches from the egg in the stomach and is propelled down the gut by peristalsis to the worm's habitat in the large in intestine.
The two main species of hookworm that infect humans, A. duodenale and N. americanus, are usually considered together, for two reasons. First, because they mostly now have an overlapping geographical distribution and occur worldwide, even if their origins were in the old and new world respectively. Second, the eggs of each species cannot be told apart when examined under a microscope, so only a diagnosis of hookworm can be made. It is necessary either to hatch the eggs and examine the larvae to tell which species is which, or to expel adult worms from the gut and recover them from the faeces. Both of these procedures are difficult and the first carries a risk of infection.
The eggs of both species of hookworm are passed in the faeces. A female A. duodenale is estimated to lay 10 000–25 000 eggs a day and a female N. americanus 5000–10 000 eggs a day (Pawlowski et al. 1991). Again, like A. lumbricoides, the average number of eggs produced per female worm declines as the number of worms increases, a mechanism believed to contribute to the stability of worm populations (Bundy 1990) but which may also serve to help prevent massive infections occurring. Hookworm larvae hatch out onto the soil within 48–96 h of being passed in the faeces, although the speed of maturation depends on the temperature and humidity (Smith 1990). The larvae do not feed, so have a finite life span measured in a few weeks, again depending on the temperature, the degree of humidity to prevent desiccation, and whether the larvae are shaded from sunlight or not, such as by vegetation (Smith 1990). Hookworm larvae are thought to survive longest on light, sandy soil rather than on heavy, clay soil, and in places where the relative humidity is high (Mabaso et al. 2003).
Infection with both species of hookworm occurs when the third‐stage larvae on the soil come into contact with bare skin. The infectious larvae burrow through the epidermis by a process of mechanical penetration facilitated by secreted protease enzymes (Salafsky et al. 1990). In large numbers this can cause an allergic reaction called ‘ground itch’ (Gilles 1990). Infections with A. duodenale can also occur if the larvae are swallowed (Schad 1990).
Once in the human body, hookworm larvae migrate through the blood system and heart to the pulmonary blood vessels, where they bore into the alveoli. The action of the cilia lining the bronchioles carries the larvae upwards, into the oesophagus, where they are swallowed, pass through the stomach and reach the small intestine. The worms take about 4–5 weeks to mature and start producing eggs, called the pre‐patent period. There is circumstantial evidence that larval worms may get into breastmilk, because hookworm eggs have been seen in the faeces of infants too young to have been exposed to larvae (Schad 1990).
The buccal cavity of both species of hookworms that infect humans contains sharp plates or ‘teeth’ used to grasp and cut gut tissue to enable the worms to suck up blood and tissue fluids (Roche & Layrisse 1966). Both species of hookworm secrete an anticoagulant to maintain the flow of blood (Roche & Layrisse 1966; Hotez & Cerami 1983). It has been estimated that a single A. duodenale causes blood loss of 0.2 mL per day (range 0.14–0.26 mL) compared with 0.04 mL per day (range 0.02–0.07 mL) by N. americanus (Roche & Layrisse 1966). When expressed in terms of the number of worms needed to lose 5 mL of blood each day, this corresponds to 25 A. duodenale and 110 N. americanus (Pawlowski et al. 1991). Some of the blood and iron ingested by hookworms is excreted into the host's gut, and is available for absorption lower down the intestine. It has been estimated that as much as 40–60% of the iron lost into the gut may be reabsorbed by anaemic people (Roche & Layrisse 1966). Whether or how quickly any given individual develops anaemia will depend on five factors: the number of worms; the duration of infection (see Section 2.3); the initial haemoglobin concentration; the size of the existing reserves of iron in the bone marrow; and, most importantly, the amount and bioavailability of iron in the diet (Gilles 1990; Crompton & Whitehead 1993).
1.3 How worms may affect human nutrition and growth
There are several mechanisms by which intestinal nematodes could affect the nutritional status of their host:
-
•
by feeding on the contents of the host's gut, including the host's secretions that make up the exoenteric circulation;
-
•
by feeding on host tissues, including blood and serum, which leads to a loss of iron and protein;
-
•
by causing maldigestion or malabsorption of nutrients;
-
•
by inflammatory responses that lead to the production of substances that may affect appetite and food intake, or substances that modify the metabolism and storage of key nutrients such as iron; and
-
•
through contingent responses to infection, such as fever, leading to an increased metabolic rate; by causing hypertrophy of muscles; and by immune responses to infection, all of which result in the diversion or use of nutrients and energy for purposes that would not have been necessary had worms not been present.
All intestinal parasites obtain their nutrients either from the food and intestinal secretions of their host, or from their host's tissues and body fluids. The nutritional needs of parasites are relatively small compared with a well‐nourished host, mainly because their relative biomass is small (see Box 2).
Box 2. The nutrition of worms
There is a common belief that worms make children thin by consuming the food in their intestine or that they increase children's weight by their presence (SC/UK 2004). This is a fallacy because the biomass of worm tissue is relatively small in comparison with the biomass of an infected child. For example, a female Ascaris lumbricoides, which is the largest intestinal nematode worm that infects humans, has an average weight of some 3.2 g and a maximum of 9.0 g. Male worms are half the size. A study of the worm burdens of 268 infected children aged 4–10 years found an average of 23 A. lumbricoides per child which weighed an average of just under 50 g. This was 0.3% of the average weight of the children. If 70% of the weight of worm tissue is metabolically active (excluding the chitinous exoskeleton and the pseudocoelomic fluid), and if the metabolic rate and need for energy of the worm is the same as its homeothermic host (77 kcal kg−1 day−1), then a biomass of 50 g would require about 2.7 kcal of energy a day. As helminths have an anaerobic metabolism which only generates about 5% of the energy as aerobic metabolism, this biomass of worms would require some 54 kcal, equivalent to 13 g of glucose, a day. However, as the metabolic by‐products of the worm's metabolism are likely to be absorbed and metabolized by the host, in whom they could produce energy, the inefficiency of energy production by worms may be mitigated. Some unique metabolites of A. lumbricoides can be detected in human urine in proportion to the number of worms in the host (Hall & Romanova 1990). These rough estimates indicate that the nutritional needs of most worm burdens are small in relation to a child host, although during a severe shortage of food, the loss of any nutrients to a moderate or large worm burden may exacerbate undernutrition.
This means that worms such as A. lumbricoides only take a relatively small proportion of the host's food from the gut. A study of tapeworms in protein malnourished rats indicated that the amount of protein in worms was only about 1% of the total protein intake, even if there were enough worms to fill the small intestine (Hall 1983). Although worms do not have an aerobic metabolism and are relatively wasteful of substrates to generate energy, it is also likely that the worm's excretory products are absorbed, metabolized and excreted by the human host.
The impact of worms’ nutritional requirements may be more significant to a host if the worms feed directly on host tissues, because the physical damage they do may have important consequences, in addition to the effects of nutrient losses resulting from feeding. For example, when hookworms move from a site at which they have been feeding, it may continue to bleed into the gut as a result of the persistent effects of the anticoagulant secreted from the worm's salivary glands (Hotez & Cerami 1983). Moderate to heavy infections with hookworm are strongly associated with anaemia (Roche & Layrisse 1966) which has consequences for growth (Stephenson et al. 1993a), physical fitness (Latham et al. 1990b; Stephenson et al. 1990; Stephenson et al. 1993a) and worker productivity (Gilgen et al. 2001; Hunt 2002; Selvaratnam et al. 2003). The feeding of hookworms can also cause a loss of blood proteins and the development of hypo‐albumenaemia (Gilles 1990).
Maldigestion and malabsorption may occur as a result of physical damage to the gut surface. The presence of moderate burdens of Ascaris suum in experimentally infected pigs has been shown to cause flattening of villi as well as villous atrophy and fusion (Martin et al. 1984), all of which could lead to a loss of brush border enzymes and a reduced surface area for digestion and absorption.
Damage to villi might be expected to lead to the loss of lactase. A study of African children infected with intestinal parasites, including A. lumbricoides, did not find evidence of lactose malabsorption (Gendrel et al. 1992) although this may be a result of a failure of the study to take into account the worm burden (see Section 2.3). Another study, of Panamanian children, did find differences between groups of infected and uninfected children in the results of hydrogen breath tests, an indicator of lactose malabsorption (Carrera et al. 1984).
Another possible cause of malabsorption could be bacterial overgrowth of the small intestine because of the presence of worms, although this is more commonly associated with infections such as G. duodenalis (Tandon et al. 1977; Farthing 1993; de Boissieu et al. 1996; Müller & von Allmen 2005).
A loss of appetite has been reported as a consequence of worm infections (Symons 1985; Hadju et al. 1996; Easton 1999) but it is hard to study, because it would mean leaving some infected children untreated while others were given an anthelmintic. However, several studies have measured improvements in the appetite of children after treating worms (Latham et al. 1990b; Stephenson et al. 1993a; Hadju et al. 1996), which has provided quite convincing evidence of an important mechanism by which worms can impair children's nutrition and growth.
The contingent responses to infection, which have been described in a previous review (Hall 1985), lead to a waste – or at least an unnecessary diversion – of resources as a result of the physical and immunological responses to infection. These are hard to quantify in humans, so experimental animals are often used. For example, experimental infection of pigs with moderate numbers of A. suum, a species very similar to A. lumbricoides that can also infect humans, has been shown to lead to an increase by 50–100% in the wet weight of the small intestine compared with uninfected controls, mainly because of hypertrophy of the tunica muscularis (Stephenson 1987). This is likely to be in response to the need for increased muscularity to push food past worms in the small intestine by peristaltic contractions. Histological cross‐sections of the mucosa also show changes in tissues in addition to the flattened villi described above: the lamina propria becomes infiltrated with mast cells and eosinophils as a result of immune reactions to the presence of worms in the intestine, while goblet cells show hyperplasia as a result of producing more mucus, perhaps to try to protect the villi from erosion (Stephenson et al. 1980a; Stephenson 1987). These may be usefully adaptive and protective responses to infection, but they represent a diversion of nutrients that should not be necessary and could be better used for growth if they happen in an already undernourished child.
1.4 Design of studies to estimate the impact of worms
The main problem with studying the impact that worms have on child growth and nutrition has been touched upon in the previous section: the need for untreated controls. If worms impair growth, and if treating worms leads to extra or catch‐up growth, then it is necessary to measure the difference that treatment makes between treated and untreated subjects, not just the absolute amount of growth that occurs after treatment. This is because some amount of growth and weight gain should occur naturally in all children, unless they are severely undernourished or have a hormonal disease.
It could be argued that it is enough to express weight gain as a change in proportion to a reference value, such as a higher z‐score of weight‐for‐height, or a greater percentage of the median value. Such improvements in anthropometric status could occur as a result of secular changes in the food supply, or as a result of better health because of seasonality in the transmission of other diseases, such as malaria and diarrhoea. Concurrent and untreated controls are essential to the validity of the conclusions of any study of the impact of treating intestinal worms on child growth and nutritional status and are an important criterion for including any study in a meta‐analysis.
1.5 Aims
The aim of this review is:
-
•
to describe the epidemiological factors that influence the impact that intestinal worms have on human nutritional status and growth;
-
•
to describe the factors that affect the impact of anthelmintic treatment; and
-
•
to undertake a meta‐analysis of the effects of intestinal worms on children's nutritional status and growth.
2. Factors affecting the impact of intestinal worms
In order to understand the impact that intestinal nematode worms have on the nutritional status and growth of children by any of the mechanisms proposed in Section 1, it is necessary to understand the factors that are likely to influence the degree or magnitude of their effects.
2.1 Species of intestinal worm
The most important species in terms of disease are A. lumbricoides, T. trichiura and the hookworms. These worms live in different parts of the intestine, differ in the route they take to reach their adult habitat and feed in different ways. This has been described in Section 1.2.
Although A. lumbricoides is undoubtedly the most common species worldwide, it is very hard to distinguish from A. suum (Crompton 1989). It is quite likely that both species occur together, especially in places where pigs are allowed to roam freely in their search for food in an environment inhabited by people (Kofie & Dipeolu 1983; Maruyama et al. 1997).
Although the two hookworm species are considered together because there is no easy way to tell them apart, there is evidence that A. duodenale is more pathogenic than N. americanus because it consumes more blood per worm (Roche & Layrisse 1966). As well as measurements of blood loss using radioactive isotopes (Roche & Layrisse 1966), there is epidemiological evidence from a study of school children in Pemba, a small Tanzanian island where both types of hookworms occur, that in schools where the prevalence of A. duodenale is high there may be more anaemia and iron deficiency than in schools where N. americanus is the predominant species (Albonico et al. 1998).
The conclusion is that different species will have different effects on the nutritional status and growth of children.
2.2 Prevalence of infection
The first important epidemiological parameter that describes the potential effect of worms on human health is the proportion infected, or prevalence. Infections are usually diagnosed by seeing the characteristic eggs of each species of worm in faeces examined under a microscope, which simply indicates that there is present in the gut at least one sexually mature female and one male worm. The exception is A. lumbricoides, because unfertilized female worms can produce infertile or ‘decorticated’ eggs; they can be identified because they are longer and narrower than fertilized eggs (Crompton 1989; WHO 1994a).
Infections with intestinal worms may therefore be missed if there are only female worms present, or only male worms, or only immature worms. Such infections are not clinically important, but they will lead to an underestimate of the prevalence.
Infections may also be missed if an insensitive method of diagnosis is used, such as a direct faecal smear, and if the concentration of eggs in faeces is low. A study in Bangladesh found that some 8% of infections with A. lumbricoides were missed when infections were diagnosed using a moderately sensitive ether sedimentation method (Hall 1981) and compared with a diagnosis made by expelling worms using an effective anthelmintic drug (Hall et al. 1999).
Table 2 shows the range in numbers of eggs estimated to be produced daily by a female worm of each species. They suggest that the sensitivity of diagnosing a light infection of a few worm varies between species, probably in the rank order A. lumbricoides, A. duodenale, T. trichiura and lastly N. americanus.
Table 2.
Estimated fecundity of fertilized females of the major species of intestinal nematode worms. Data from Sinniah (1982), Bundy & Cooper (1989) and Anderson & May (1991). The numbers are based on very few data and do not take into account density‐dependent fecundity or geographical variation in fecundity. At best they can be considered as indicative of the relative orders of magnitude of egg production
| Species | Eggs per female worm |
|---|---|
| Ascaris lumbricoides | <200 000 |
| Trichuris trichiura | 3 000–20 000 |
| Ancylostoma duodenale | 10 000–20 000 |
| Necator americanus | 3 000–6 000 |
Infections with the three main types of intestinal worms number among the most common infections of children in the world today. Table 3 presents some recent estimates of the numbers of people infected with A. lumbricoides, T. trichiura and the hookworms, by age range. Table 4 presents recent estimates of the prevalence of these infections in young school‐age children, aged 5–9 years. This age group is particularly likely to have moderate to heavy infections and is vulnerable to their impact on nutritional status.
Table 3.
Estimates of the population and number of people infected in millions, and the prevalence of infection with the main types of intestinal nematodes by region and age group. Data extracted from de Silva et al. (2003)
| Population | Age range (years) | Total | % infected | ||||
|---|---|---|---|---|---|---|---|
| 0–4 | 5–9 | 10–14 | ≥15 | ||||
| Ascaris lumbricoides | |||||||
| Latin American and Caribbean | 530 | 8 | 10 | 10 | 56 | 84 | 15.8 |
| Sub‐Saharan Africa | 683 | 28 | 28 | 25 | 92 | 173 | 25.3 |
| Middle east and north Africa | 313 | 3 | 3 | 3 | 14 | 23 | 7.3 |
| South Asia | 363 | 13 | 15 | 13 | 56 | 97 | 26.7 |
| India | 1027 | 15 | 18 | 17 | 89 | 139 | 13.5 |
| East Asia and the Pacific | 564 | 20 | 25 | 25 | 134 | 204 | 36.2 |
| China | 1295 | 35 | 44 | 51 | 371 | 501 | 38.7 |
| Total | 4775 | 122 | 143 | 144 | 812 | 1221 | 25.6 |
| Trichuris trichiura | |||||||
| Latin American and Caribbean | 530 | 10 | 12 | 12 | 66 | 100 | 18.9 |
| Sub‐Saharan Africa | 683 | 26 | 27 | 23 | 86 | 162 | 23.7 |
| Middle east and north Africa | 313 | 1 | 1 | 1 | 4 | 7 | 2.2 |
| South Asia | 363 | 10 | 11 | 10 | 43 | 74 | 20.4 |
| India | 1027 | 8 | 9 | 9 | 47 | 73 | 7.1 |
| East Asia and the Pacific | 564 | 16 | 19 | 19 | 105 | 159 | 28.2 |
| China | 1295 | 15 | 19 | 22 | 163 | 219 | 16.9 |
| Total | 4775 | 86 | 98 | 96 | 812 | 1092 | 22.9 |
| Hookworm | |||||||
| Latin American and Caribbean | 530 | 1 | 3 | 5 | 41 | 50 | 9.4 |
| Sub‐Saharan Africa | 683 | 9 | 18 | 29 | 142 | 198 | 29.0 |
| Middle east and north Africa | 313 | 0 | 1 | 1 | 8 | 10 | 3.2 |
| South Asia | 363 | 2 | 5 | 8 | 44 | 59 | 16.3 |
| India | 1027 | 2 | 5 | 8 | 56 | 71 | 6.9 |
| East Asia and the Pacific | 564 | 4 | 9 | 16 | 120 | 149 | 26.4 |
| China | 1295 | 3 | 9 | 18 | 173 | 203 | 15.7 |
| Total | 4775 | 21 | 50 | 85 | 584 | 740 | 15.5 |
Table 4.
The estimated number and percentage of children aged 5–9 years infected with the three main species of soil‐transmitted helminths and the population at risk in 2002 derived from de Silva et al. (2003) and A. Hall & N.R. de Silva (unpublished data) in the main geographic areas covered by the World Health Organization. Data for some countries are missing. The estimate for the number and percentage infected with any of these three species has assumed that where they are endemic, they occur in the same populations and that the chances of being concurrently infected with two or more species are independent
| World Bank regions | Population | Ascaris lumbricoides | Trichuris trichiura | Hookworms | Any species | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. × 106 | No. × 106 | % | No. × 106 | % | No. × 106 | % | No. × 106 | % | |
| Asia and West Pacific | 124.2 | 56.3 | 45.3 | 27.6 | 22.3 | 12.3 | 9.9 | 76.6 | 61.7 |
| South‐east Asia B | 28.4 | 9.6 | 33.6 | 9.6 | 33.9 | 5.3 | 18.6 | 18.3 | 64.3 |
| South‐east Asia D | 147.3 | 33.0 | 22.4 | 21.2 | 14.4 | 9.1 | 6.2 | 55.5 | 37.7 |
| Americas B | 45.0 | 7.0 | 15.5 | 8.8 | 19.5 | 2.2 | 4.8 | 15.8 | 35.2 |
| Americas D | 9.1 | 3.4 | 37.7 | 3.2 | 35.0 | 0.9 | 9.5 | 5.8 | 63.3 |
| Middle east B | 15.3 | 1.0 | 6.7 | 0.0 | 0.3 | 0.0 | 0.2 | 1.1 | 7.1 |
| Middle east D | 47.2 | 5.0 | 10.6 | 1.4 | 3.0 | 1.2 | 2.6 | 7.3 | 15.5 |
| Africa D | 44.6 | 13.3 | 29.7 | 10.0 | 22.3 | 7.6 | 17.0 | 24.4 | 54.7 |
| Africa E | 51.9 | 14.8 | 28.6 | 16.9 | 32.5 | 10.1 | 19.4 | 31.7 | 61.1 |
| Total | 513.0 | 143.4 | 27.9 | 98.7 | 19.2 | 48.6 | 9.5 | 242.8 | 47.3 |
Figure 2 shows the typical relationship between age and the prevalence of infection with A. lumbricoides derived from a study of people living in an urban slum in Bangladesh; a similar relationship is commonly observed for T. trichiura. Figure 2 indicates that infections are acquired in the first 2 years of life and that around 80% of all age classes are infected, a high but typical proportion.
Figure 2.
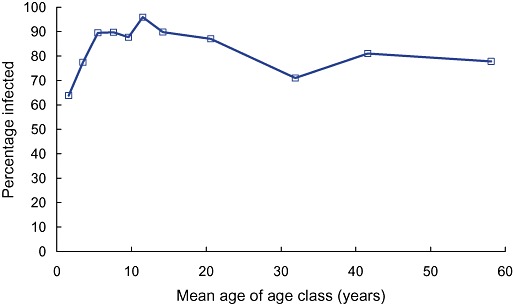
The prevalence of infection with Ascaris lumbricoides in 11 age classes of males living in an urban slum in Dhaka, Bangladesh (data from Hall et al. 1999).
Hookworms tend to show a different pattern in which the prevalence typically increases with age, reaching a peak in late adolescence and adulthood (Bundy et al. 1992a). The reason for this difference is not clear, especially as children are less likely to wear shoes than adults and so could be considered to be more to be exposed to hookworm larvae on the soil. The differences in prevalence may reflect where worms are transmitted in what have been called ‘domains of infection’ (Cairncross et al. 1996). The transmission of both A. lumbricoides and T. trichiura is thought to occur within or around the household, in the domestic domain, while hookworms may be transmitted beyond the household, in the public domain which is frequented more by adults than children (Cairncross et al. 1996).
Although the prevalence of infection in children may provide some indication of the importance of each worm in terms of health and nutrition, there is no clear threshold prevalence associated with disease, largely because the relationship between prevalence and the intensity of infection is strikingly non‐linear. Figure 3 shows the relationship between the prevalence of infection with intestinal worms and the worm burden, derived from data collected in Bangladesh on A. lumbricoides (Hall et al. 1999). It can be seen that, below a prevalence of about 50%, the mean worm burden is relatively low, but rises almost exponentially above a prevalence of 60%. This means that even a prevalence of up to about 50% is associated with a low average worm burden, and that the prevalence of infection is a poor indicator of the probability of disease unless it is 70% or greater. This relationship helps to explain the use of a threshold prevalence of 50% for administering mass anthelmintic treatment for both soil‐transmitted helminths and schistosomiasis, a threshold that was endorsed by a World Health Organization (WHO) Expert Committee in 2001 (WHO 2002a). This threshold has subsequently been lowered by the WHO to 20% for mass treatment once a year in what they classify as ‘low‐risk’ communities, and the WHO now apply the 50% threshold to define a ‘high‐risk’ community where mass treatment twice a year is warranted (WHO 2006).
Figure 3.
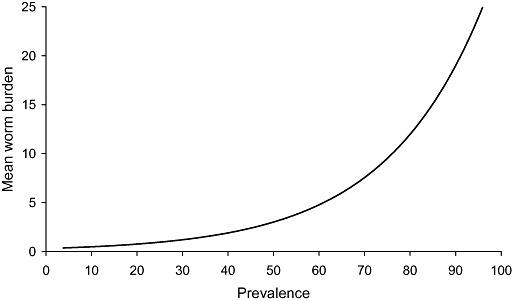
The typical relationship between the prevalence and the mean worm burden for intestinal nematode worms (see Guyatt et al. 1990). The shape of the curve is derived from data collected during a study of Ascaris lumbricoides in Bangladesh (Hall et al. 1999).
The relationship between prevalence and disease is further complicated by the mix of species: in many parts of the world it is common to find all three major types of worms together, so that some children have two or three infections. Although the prevalence of A. lumbricoides and T. trichiura is often correlated (Booth & Bundy 1992), it seems that an infection with one species does not predispose to the presence of another. Figure 4 shows a diagrammatic representation of the percentage of individuals who have multiple infections when the prevalence of A. lumbricoides is 60%, T. trichiura is 50% and the hookworms is 40%, all arbitrary but typical figures. If the probability of having one infection is independent of having another, then it can be estimated that 32% of individuals have at least two infections and 18% have all three infections (Fig. 4).
Figure 4.
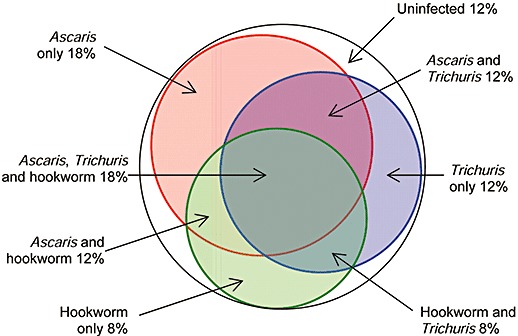
A diagrammatic representation of the proportions of a population of 100% who are infected with one, two or three types of worms when the prevalence of infection with Ascaris lumbricoides is 60%, Trichuris trichiura is 50% and hookworm is 40%. It is assumed that the probability of each infection is independent of each other and that probability of having two or more infections is multiplicative.
There is no method to assess the impact of multiple infections: they could be additive but might be multiplicative or even antagonistic if species occur in the same location in the gut, such as A. lumbricoides and the hookworms. As multiple infections may be as common as or more common than single infections, it is hard to estimate the relative benefit of treating each different species, especially as the drugs used to treat intestinal nematode worms are effective against all species, if to differing degrees (see Section 3.2).
In conclusion, the WHO threshold of 50% infection provides a reasonable basis for applying mass treatment. Below this threshold few people have moderate to heavy worm burdens that cause disease and the majority are uninfected; above this threshold the likelihood of moderate to heavy infections increases exponentially. This is not to say that worms do not cause disease below a prevalence of 50%, but the effect on a very small minority may be lost in the group average. A prevalence of 50% was taken as a minimum for studies of deworming to be included in the meta‐analysis reported below, and studies such as those of Garg et al. (2002) in which the prevalence of infection with any worm before treatment with an anthelmintic was only 11% were excluded. This threshold may seem somewhat arbitrary, but it is based on the relationship shown in Fig. 3 and it would be unusual to include any uninfected individuals in a trial of a drug to treat a bacterial diseases. A prevalence of 50–100% represents a typical range in many human communities in which mass anthelmintic treatment is given, and the effectiveness of treatment in such studies represent common epidemiological circumstances.
2.3 Number and distribution of worms
Each worm that becomes established in a host represents the successful hatching, migration, establishment and development of a single fertile egg. Nematode worms do not multiply within their host, except for S. stercoralis (see above) the eggs of which can hatch within the lower bowel to release infective filariform larvae that penetrate the gut wall in a process called autoinfection (Schad 1989). This explains the persistence of S. stercoralis in British soldiers who were prisoners of the Japanese in Asia during the second world war and who have developed strongyloidiasis in their old age (Gill & Bell 1979, 1987; Gill et al. 2004).
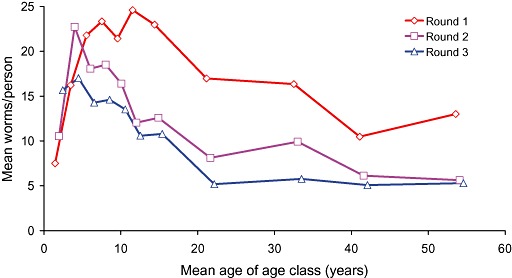
For each of the four major types of intestinal worms the probability of disease is related to the number of worms in the host, called the worm burden. Figure 5 shows the average number of A. lumbricoides recovered from males in 11 age classes in an urban slum in Bangladesh who were given a drug that paralysed their worms so that they were expelled by peristalsis, then recovered, washed and counted. It shows that the heaviest average infections were found in school‐age children from 5 to 15 years old, a characteristic typical of many helminth infections. The notable exception is the hookworms, for which the prevalence and mean worm burden tend to increase with age so that adolescents and adults tend to be most heavily infected (Bundy 1990; Bradley et al. 1992).
Figure 5.
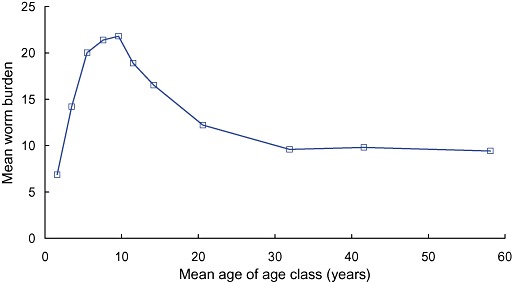
The average number of worms recovered from males in 11 age classes living in an urban slum in Bangladesh (Hall et al. 1999).
There are two theories to explain the shape of the distribution in Fig. 5. First, it could reflect differences in behaviour, because children are more exposed to worm eggs than adults, perhaps as a result of playing on faecally contaminated ground and poor personal hygiene.
Second, it could reflect the development of a partially effective immune response to infection, or perhaps a more effective immune response in some individuals than others as a result of repeated exposure to worm larvae. The study in Bangladesh however found a statistically significant difference in the mean worm burden of A. lumbricoides between adult males (who leave their community to work during the day) and adult females (who stay at home in the crowded, unsanitary environment), which suggests that exposure more than immunity influences this distribution (Hall et al. 1999).
When the distribution of worms among hosts is examined it is typical to find that it is highly skewed, so that most individuals have light infections while a minority have moderate to heavy infections. Figure 6 illustrates this distribution using data on the number of A. lumbricoides recovered from 1765 people who were treated with pyrantel pamoate to paralyse and expel their worms. The distribution shown in Fig. 6 is best described by the negative binomial, which is purely an empirical fit, and implies nothing about the biological reason why worms should be distributed in this way. This distribution, which is described as aggregated or overdispersed, is typical of most helminth infections and has been observed even in pigs each of which has been infected experimentally with the same number of fertile eggs of A. suum (Stephenson et al. 1980a; Boes et al. 1998). This suggests that the distribution derives from differences between hosts in the establishment of worms rather than in differences in exposure to eggs.
Figure 6.
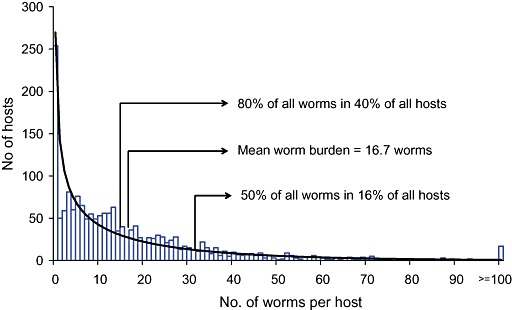
The distribution of Ascaris lumbricoides in 1765 people living in an urban slum in Dhaka, Bangladesh (Hall et al. 1999). The black line shows the negative binomial distribution.
The equation that describes this distribution is defined by three parameters, the prevalence (P), the arithmetic mean worm burden (M), and k, a parameter that varies inversely with the degree of aggregation or clumping of worms in a few hosts, so that:
The parameter k captures the degree to which worms in a small proportion of hosts tend to be aggregated, clumped or overdispersed (terms commonly used in this context). Small values of k, typically less than 1, indicate a high degree of aggregation of worms, irrespective of age, sex or any other factor. The study in Bangladesh indicated that k varied with the mean worm burden so that, as the mean burden increased, the degree of aggregation decreased (Hall et al. 1999). The implication is that, when the average worm burden is large, more people are likely to have moderate to heavy infections and may be diseased as worms are less aggregated. Figure 7 shows the cumulative percentage of all worms recovered from 1765 people in urban Dhaka plotted against the cumulative percentage of hosts, for worm burdens between zero and 187 worms. It shows that a half of all subjects expelled only 10% of all worms, and that 80% of all subjects contained only 40% of all worms. The remaining 60% of worms were recovered from only 20% of individuals.
Figure 7.
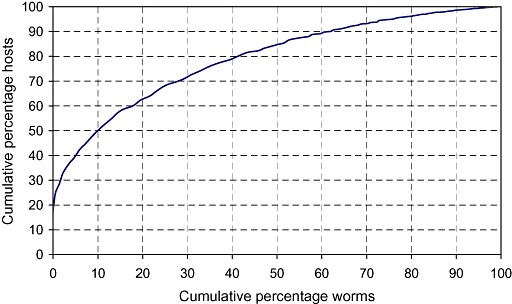
The cumulative percentage of worms recovered from 1511 people infected with Ascaris lumbricoides in an urban slum in Bangladesh ranked according to worm burdens from zero to the maximum of 187 worms, plotted against the cumulative number of subjects from whom they were recovered (data from Hall et al. 1999).
If it is typical to find that a small proportion of people harbour a large proportion of worms, what happens when they are treated? Evidence from studies of reinfection after treatment shows that heavily infected individuals tended to become heavily reinfected again while lightly infected people become lightly reinfected, leading to the theory that some individuals are predisposed to infection and others are not (Haswell‐Elkins et al. 1987; Bundy & Cooper 1988; Holland et al. 1989; Forrester et al. 1990; Chan et al. 1994; Kightlinger et al. 1995). If this is so, could efforts be concentrated on the individuals who were predisposed to worms? This was examined in a study of infection and reinfection with A. lumbricoides in 880 individuals in Bangladesh (Hall et al. 1992). It was found that, although there was evidence of a predisposition to moderate to heavy infections, over three rounds of treatment and two periods of reinfection of 6 months, about two‐thirds of all subjects were moderately or heavily infected at least once (Hall et al. 1992). This suggested that there was no benefit in identifying moderately to heavily infected people on the assumption that they were predisposed individuals, and mass treatment should be given rather than any form of selective treatment.
The biological implications of the aggregation of worms are that most individuals in a community at any one time have light infections, but a minority ranging from <1% to 40% will have moderate to heavy infections and are most likely to be diseased. If treatment is given periodically and reinfection occurs (which will happen because eggs can persist in the environment for many months, if not years), then over a period of 2–3 years a majority of individuals will be moderately to heavily infected at some point. This has implications for measuring the impact of mass treatment, as a minority will benefit more than the majority in the short term, but over a longer period of periodic treatment, an increasingly larger proportion will benefit.
A light infection probably has little effect on the nutritional status of a host, and the worm burden is the key indicator of the probability of disease. This requires that worms are expelled and counted, something that is difficult to do, and it destroys the worm burden at the same time, so that infected subjects can no longer be followed. So in most surveys of worms the concentration of eggs in faeces is used as an indicator of the intensity of infection. However, the perception of egg counts tends to be relative, so that what is seen to be a moderate egg count in one place could be considered to be high or even low in another. To provide some guidance on assessing egg counts a WHO Expert Committee in 1987 suggested threshold egg counts to classify light, moderate and heavy infections with A. lumbricoides and T. trichiura (WHO 1987). In 2002 a new Expert Committee added hookworm to the list although the previous committee had stated that egg counts for this worm could not be given because the ‘critical worm load differs locally depending on age, sex, iron intake and species of hookworm’ (WHO 2002a). These thresholds are shown in Table 5.
Table 5.
The threshold concentrations of worm eggs in faeces used to classify infections as light, moderate and heavy proposed by a World Health Organization Expert Committee in 2002 (WHO 2002a). No references or data are given in support of these numbers
| Intensity | Ascaris lumbricoides | Trichuris trichiura | Hookworms |
|---|---|---|---|
| Low | 1–4 999 | 1–999 | 1–1 999 |
| Moderate | 5 000–49 999 | 1 000–9 999 | 2 000–3 999 |
| High | ≥50 000 | ≥10 000 | ≥4 000 |
Since the Expert Meeting in 1987 it has been clearly shown for A. lumbricoides at least that the relationship between worm burden and egg count is both non‐linear and differs between worms in different countries (Hall & Holland 2000). For example, 20 worms was associated with around 1300 eggs per gram of faeces or 2300 egg g−1 in two studies in Bangladesh, with 17 300 eggs g−1 in Iran, with 22 000 eggs g−1 in Nigeria and Madagascar, with 27 000 eggs g−1 in Burma and with 44 500 eggs g−1 in Mexico (Hall & Holland 2000). This indicates that there is no consistent relationship between egg counts and worm burdens that can be applied universally, for A. lumbricoides at least.
2.4 Duration of infection
One of the main factors besides the worm burden that is likely to contribute to the development of disease is the duration of infection, something that is not usually known. Table 6 shows the estimated life span of the major species of intestinal nematodes of humans.
Table 6.
Estimates of the life span of the major species of intestinal nematode worms of humans (data from Anderson & May 1991)
| Species | Life span (years) |
|---|---|
| Ascaris lumbricoides | 1–2 |
| Trichuris trichiura | 1–2 |
| Ancylostoma duodenale | 2–3 |
| Necator americanus | 2–3 |
| Enterobius vermicularis | <1 |
| Strongyloides stercoralis | >50* |
Resulting from autoinfection.
But even if a worm such as A. lumbricoides can live for as long as 2 years and is then expelled from the gut when it dies, new worms are continually acquired so that people remain persistently infected with slowly changing numbers of worms. Figure 2 indicates that just over 60% of children aged 1–2 years in the study in Bangladesh were infected with A. lumbricoides and Fig. 5 indicates that they contained an average of about seven worms. As both the prevalence and mean worm burden are higher in older age groups, it suggests that most people are infected throughout their life.
2.5 Rate of reinfection
If the population of worms in a community of hosts is perturbed by giving mass treatment with an anthelmintic, reinfection can occur immediately and the number of worms will rebound to a similar number as before, a state called equilibrium. Without treatment there may be fluctuations in time in the numbers of worms in individuals as some die and others are gained, but the number of worms in the community seems to reach a relatively steady state, perhaps driven by factors such as sanitation, the contamination of the environment, behaviours that put people at risk and environmental conditions that favour the survival of infectious stages. Figure 8 shows the prevalence of infection with A. lumbricoides in 880 people at three rounds of treatment, 6 months apart and Fig. 9 shows the mean worm burden on the same occasions (Hall et al. 1992).
Figure 8.
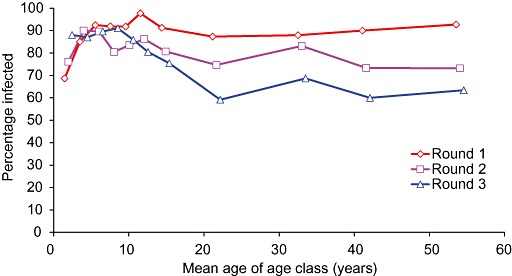
The prevalence of infection with Ascaris lumbricoides at three rounds of treatment 6 months apart (Hall et al. 1992).
Figure 9.
The mean worm burden with Ascaris lumbricoides at three rounds of treatment 6 months apart (Hall et al. 1992).
The prevalence, shown in Fig. 8, rebounded very quickly after treatments in the school‐age children, perhaps because the force of infection was greater in this age class, but was lower at each round in the four adult age classes. The mean worm burden shown in Fig. 9 was, however, lower at each round of treatment in all age classes except for the very youngest cohort, whose exposure to worm eggs probably increased as they became mobile and were increasingly exposed to worm eggs. 8, 9 show that periodic treatment may do little to perturb the prevalence of infection but, if given often enough, it can help to prevent reinfection with the same number of worms. In an environment such as an urban slum in Bangladesh, it may be necessary to give treatment for worms at least three times a year rather than twice annually, in order to sustain low worm burdens and reduce the probability of disease.
8, 9 also illustrate that the prevalence is a poor indicator of the effectiveness of worm control by periodic deworming, and that an indicator of the intensity of infection is better (Bundy et al. 1992a). Ideally both should be measured during a programme of mass deworming.
2.6 Summary
The probability that an infected child has disease or malnutrition caused by intestinal worms is related to:
-
•
the species of worm;
-
•
the mixture of species;
-
•
the worm burden of each species; and
-
•
the duration of infection before treatment.
The number of worms is difficult to assess without expelling them from the gut. The concentration of eggs in faeces may only represent a worm burden in a specific locality, because fecundity varies with the number of worms and perhaps between strains of worms in different locations around the world. There are no threshold numbers of worms to classify burdens as light, moderate or heavy. There are no methods to combine the probability of disease because of different numbers of worms of different species in the same individual.
3. Factors affecting the impact of treatment
Section 2 has described how the biology of each species of worm and the distribution of worms among hosts is likely to influence the effect that they have on the growth and nutritional status of children. As it is unethical to infect children with worms and measure prospectively their impact on growth, evidence of the effect of worms on humans comes either from cross‐sectional surveys or from experimental studies in which changes in key indicators of health, growth and nutrition are measured after giving treatment.
Cross‐sectional studies are difficult to interpret. Although several have shown an association between infections with intestinal worms and undernutrition or stunted growth (Cerf et al. 1981; Egger et al. 1990; Gupta 1990; Saldiva et al. 1999; Moore et al. 2001; Muniz et al. 2002; Al‐Mekhlafi et al. 2005), other studies have not (Pegelow et al. 1997) or have had mixed results (Mahendra Raj et al. 1997b). The best‐analysed studies have controlled for factors such as age, sex and socio‐economic status, and have examined associations not just with the presence of infection, but with an estimate of the intensity of infection. The main problem with doing such analyses lies in the lack of data on the actual worm burden rather than on egg counts, which do not have a consistent relationship with worm burden, and with quantifying the combined effects of more than one species of worm.
There is also considerable potential for publication bias in cross‐sectional studies because only interesting and biologically plausible associations are likely to be written up and submitted for publication, while negative findings are either not submitted or are less likely to be accepted by a scientific journal.
But the main scientific problem with cross‐sectional studies is related to temporality: did malnutrition predispose children to infection with worms, or did worms cause the malnutrition? It is also unlikely that worms, and only worms, are responsible for all undernutrition or stunted growth. Worms are associated with poverty and a poor diet, so if worms have a harmful effect, it is likely to be in addition to underlying chronic malnutrition.
Second, infection with worms reflects exposure to human faeces, a waste material that contains many other pathogens in addition to worm eggs, such as viruses, bacteria and protozoa. Repeated episodes of diarrhoea and other infectious illnesses associated with living in an impoverished and unhygienic environment can also affect nutritional status through their effects on appetite, absorption and metabolic rate, as the studies of Mata and colleagues in Guatemala vividly demonstrated (Mata 1978). Infection with worms is an indicator of life in an environment contaminated with human faeces.
These complexities mean that it is not adequate simply to compare the growth of a group of infected children after treatment with a control group who were not infected with worms (Mahendra Raj et al. 1997a; Mahendra Raj & Naing 1998), nor is it adequate to explain the magnitude of weight gain after treatment in terms of prior egg counts (Stephenson et al. 1980b).
The need for untreated controls is reinforced when it comes to measuring the impact of treatment on weight gain or growth. Even when children are chronically undernourished, some growth can be achieved, so without a control group it is not possible to know whether a treatment has led to extra weight gain or extra growth over any given period. Making comparisons with a reference population to assess the degree of change in undernutrition will not provide an answer either. Even if an improvement in anthropometric indices is recorded, or a fall in the percentage of children who are in some way undernourished, such changes can occur as a result of secular improvements in food supply or a decrease in the transmission of other diseases such as malaria or diarrhoea over the period of a study.
This section describes factors that influence the impact of treatment on children's growth and nutritional status.
3.1 Study design: controls and randomization
In order to estimate reliably the magnitude and statistical significance of the effect of a treatment on growth or nutritional outcome, it is necessary to have an untreated control group (Stephenson 1987). To ensure that naturally occurring differences between subjects are evenly distributed between groups before treatment is given, individuals also need to be randomly assigned to each study group. As there can be large differences between individuals in the intensity of infection because of the aggregated distribution of worms, if the sample size is small then subjects may need to be stratified first by egg count, before random allocation. If the sample size is large and the prevalence is high, then random allocation is likely to be sufficient.
If the treatments are allocated by clusters, such as villages or schools rather than to individuals, there must be a sufficient number of clusters to distribute any variation between clusters in the prevalence and intensity of infections evenly between study groups. Randomized cluster designs have the potential to provide the large sample sizes that are needed to be able to detect the effects of treatment on the minority of subjects who will benefit most from treatment.
Ideally all subjects in a control group in a simple randomized trial should be given an identical placebo. This is less easy to do in cluster randomized trials, especially if anthelmintics are given through the health system. Effectiveness trials, such as the one carried out as a part of Child Health Fairs in Uganda (Alderman et al. 2006), typically have untreated controls, but they usually receive nothing rather than a placebo. This opens the study to potential bias because of self‐treatment with anthelmintics among the control group.
3.2 Anthelmintic drugs and other treatments
The drugs used to treat infections with intestinal nematodes can be divided into two main types:
-
•
drugs that act on the nervous system to paralyse worms so that they are expelled from the gut by normal peristalsis, such as piperazine, levamisole and pyrantel pamoate; and
-
•
drugs that inhibit metabolic processes, such as the benzimidazole derivatives albendazole, mebendazole and tiabendazole, which block the uptake of glucose by microtubules in the mitochondria of worms (Horton 2000, 2002), and the relatively new treatment nitazoxanide, which acts in protozoa by inhibiting the enzyme pyruvate ferredoxin oxidoreductase (Sisson et al. 2002; White 2003; Esposito et al. 2005).
There are differences between these types of drug in the efficacy with which they treat each species of intestinal worm (de Silva et al. 1997). Efficacy is assessed in two ways:
-
•
as the cure rate, which is the percentage of infected subjects in whose faeces worm eggs are no longer found after treatment (although some immature female or male worms could still remain, thereby overestimating the cure rate); and
-
•
as the egg reduction rate, which is the percentage reduction in the arithmetic or geometric mean concentration of eggs in the faeces of infected individuals. The concentration of eggs reflects the number of female worms, and an even sex ratio is usually assumed.
The cure rate and egg reduction rate are typically estimated by collecting and examining under a microscope a faecal sample collected a few days before treatment and then again some 14–21 days afterwards. The gap after treatment is advisable because there is evidence that the drugs may temporarily inhibit egg production by worms that have survived treatment (Hall & Nahar 1994).
Because of their different modes of action the two types of anthelmintic drugs differ in their efficacy: drugs that paralyse worms tend to be less effective than those that inhibit metabolic processes, perhaps because the paralysis can wear off more quickly than the effects of a metabolic inhibitor, especially if worms are anchored to the gut wall. (If A. lumbricoides are expelled from the gut using a drug such as pyrantel or levamisole and placed in warm saline, they can recover their motility after a few hours, showing the transitory effect of the paralysis).
Table 7 gives a rough guide to the range in efficacy of each type of drug against the main species of intestinal nematode worms at the usual dosage given. Data are generally lacking for the drugs that paralyse worms, because they are older and less well studied than the highly effective benzimidazole drugs, mebendazole and albendazole, which superseded levamisole and pyrantel in 1975 and 1980 respectively (Horton 2003b). In general the benzimidazoles are less effective against T. trichiura than against A. lumbricoides and the hookworms, and are less effective against hookworm than against A. lumbricoides.
Table 7.
The typical range in cure rates of drugs to treat intestinal nematode infections. The doses are those recommended by the World Health Organization (WHO 1995). The data for the drugs that paralyse worms have been derived from Huq et al. (1982), Gustafsson et al.(1987) and Albonico et al. (2003). The data for the metabolic inhibitors are summarized from Bennett (2000), and represent the approximate interquartile range in an analysis of multiple studies of drug efficacy. The data have been combined for the metabolic inhibitors albendazole and mebendazole, except for hookworm, for which there is a difference between species in efficacy
| Species | Drugs that paralyse | Metabolic inhibitors | ||
|---|---|---|---|---|
| Levamisole, 40 mg | Albendazole, 400 mg | |||
| Piperazine, 75 mg kg−1 | Mebendazole, 500 mg | |||
| Pyrantel pamoate, 10 mg kg−1 | Tiabendazole, 25 mg kg−1, 3 days | |||
| Cure rate | ERR | Cure rate | ERR | |
| Ascaris lumbricoides | 80–100% | 95–100% | 95–99% | 99–100% |
| Ancylostoma duodenale | 80–100% | 50–70% | 70–90%* | 75–95%* |
| Necator americanus | 60–90% | 20–80% † | 55–95% † | |
| Trichuris trichiura | Poor ‡ | 20–60% | 10–70% | 50–90% |
Albendazole 400 mg;
Mebendazole 500 mg;
‡ Unless pyrantel given with oxantel. ERR, egg reduction rate.
There also seem to be differences in the efficacy of the two benzimidazole anthelmintics, even though they are chemically similar. Table 8 shows data from a large study in Zanzibar of treating intestinal nematode infections with albendazole or mebendazole, which revealed statistically significant differences in the efficacy of treating hookworm (Albonico et al. 1994).
Table 8.
Data from a study in Zanzibar of the efficacy of treating intestinal nematode infections with either albendazole or mebendazole (Albonico et al. 1994). Even though the drugs have a very similar mode of action there were statistically significant differences in the cure rates and egg reduction rates, particularly for hookworms
| Species | Drug | n | Cure rate | Egg reduction rate |
|---|---|---|---|---|
| Ascaris lumbricoides | Albendazole | 1174 | 98.9 | 99.6 |
| Mebendazole | 1120 | 97.8 | 99.3 | |
| Hookworms | Albendazole | 1174 | 56.8*** | 97.7*** |
| Mebendazole | 1120 | 22.4 | 82.4 | |
| Trichuris trichiura | Albendazole | 1174 | 10.5** | 73.3*** |
| Mebendazole | 1120 | 14.2 | 81.6 |
P < 0.01;
P < 0.001.
A reason for the lower efficacy of drugs to treat hookworm compared with A. lumbricoides may be that hookworms are usually physically attached to the gut and may be better able to resist the transient effects of anthelmintic drugs than A. lumbricoides which can only maintain their position in the gut by actively swimming against the intestinal flow.
The reason for the lower efficacy of T. trichiura compared with both A. lumbricoides and the hookworms may be that they live in the large intestine, where anthelmintic drugs may be more dilute than in the small intestine. Adult T. trichiura are also typically embedded in the gut wall by their whip‐like anterior end, which may provide an anchor so that they, like hookworms, can withstand any transitory effects of anthelmintic drugs. A study in Bangladesh showed that, in order to achieve a cure rate for T. trichiura of 80%, 400 mg of albendazole had to be given daily for three consecutive days (Hall & Nahar 1994).
There is also evidence that there may be a lower cure rate for T. trichiura in Asia than in Africa: an analysis of trials of albendazole showed a median cure rate of 33% in Asia compared with 61% in Africa (Bennett 2000).
There is obvious concern that repeated mass treatment could lead to the development of drug resistance, something that has been shown to develop as a result of repeated treatment (Albonico et al. 2003). There are substantial lessons to be learned from veterinary medicine in which the same anthelmintic drugs have been used for many years and far more intensively than in humans (Coles 1995). Strategies to avoid or delay the development of drug resistance should be applied (WHO 2002a), such as alternating drugs with different modes of action, and the efficacy of treatment should be assessed periodically at sentinel sites during mass treatment programmes, the interval to depend on the frequency of treatment (WHO 2002a).
The main limitation to alternating treatments has been the lack of effective alternatives to the albendazole and mebendazole, as single‐dose treatments. One possibility is a combination of pyrantel and oxantel which has been shown to be quite effective in comparison with mebendazole (Rim et al. 1975; Albonico et al. 2002), but this mixture is not widely available. A combination of albendazole and ivermectin has been tested as a treatment for lymphatic filariasis (1999a, 1999b; Horton et al. 2000; Belizario et al. 2003) and has effects on the major species of soil‐transmitted helminths as well. The advantage of giving two drugs in combination is that the chance of genes that confer resistance to both treatments at the same time is greatly reduced.
A relatively new drug called nitazoxanide has been shown to be an effective treatment for all major species of intestinal nematode worms as well as Hymenolepis spp. (Romero Cabello et al. 1997; Juan et al. 2002). The drug currently has to be given twice a day for 3 days whereas the others are single‐dose treatments, which ensures compliance in mass treatment campaigns. The major advantage of nitazoxanide is that it is an effective treatment for intestinal protozoa, including G. duodenalis and Cryptosporidium spp., as well as anaerobic bacteria such as Helicobacter pylori and Clostridium difficile (Megraud et al. 1998; Bobak 2006). Such a drug could have a significant impact on growth and nutritional status, as studies of giving metronidazole, another broad‐spectrum antibacterial and antiprotozoal drug have suggested (Gupta & Urrutia 1982; Khin Maung et al. 1990).
The differences in the efficacy of treatments for intestinal nematode worms will therefore depend on the drug, the dose of drug, the species of worm and the strain of the worm perhaps, which will all have consequences for the impact of treatment on nutritional status and growth. In some studies included in this review a single treatment was given, while in others repeated treatment was given using the same drug. In one study included in the review, two different drugs were given: pyrantel pamoate at the first treatment and then mebendazole for subsequent bimonthly treatments (Northrop‐Clewes et al. 2001).
The situation is further complicated if anthelmintic drugs to treat other types of worms are given, such as praziquantel or metrifonate to treat infections with Schistosoma species. This is a genus of blood flukes found in Africa, the Middle East, Asia and South America, often concurrently with infections with intestinal nematode worms. Schistosoma haematobium causes bleeding into the urinary bladder as a result of the passage of the sharp‐spined eggs through tissues, while the passage of the eggs of S. mansoni and S. japonicum through the gut wall also causes internal bleeding. Studies of the impact of treatment in areas in which schistosomes occur tend to give a drug to treat these infections, as well as a drug to treat intestinal nematode worms (Beasley et al. 1999; Friis et al. 2003). In addition to treating schistosomes, the drug metrifonate is also an effective treatment for hookworm (Kurz et al. 1986), which complicates studies and measuring outcomes resulting from treatment.
Finally, if any study is to assess the impact of treating intestinal worms alone, then additional treatments cannot be given unless a factorial design is used and an untreated control group is provided. One study of the effect of treating worms with mebendazole also gave all subjects metronidazole as well, a broad‐spectrum antibacterial drug that also treats infections with intestinal protozoa (Marinho et al. 1991). This meant that the effect of mebendazole could not be separated from the effect of metronidazole. In other studies supplements of micronutrients or food were also provided, which also may have had independent effects on nutritional status and growth so making it impossible to estimate the impact of deworming alone (Latham et al. 1990a; Majumdar et al. 2003; Lind et al. 2004).
3.3 Intervals between treatments
In an infected human population the death rate of worms tends to a reach a balance with the rate at which worms are acquired, so an equilibrium develops. The effect of mass treatment is to cure a proportion of people, which reduces the prevalence of infection, and treatment expels a proportion of the total worm population, which reduces the mean worm burden. As there is no fully protective immunity to worms, reinfection can occur immediately, especially as infective eggs can survive in the environment for months in suitable conditions. The rate of reinfection is strongly related to the prior prevalence and mean worm burden, as well as to local sanitation, which influences the number of infective stages in the environment. Studies of reinfection after treatment and mathematical models that simulate treatment and reinfection have shown that the prevalence of infection can rebound within a few months after treatment, but the worm burden takes considerably longer to reach the pre‐existing number (Hall et al. 1992). This means that the prevalence may show only a small difference between rounds of treatment, but the mean worm burden may decline.
Figure 10 shows data on the prevalence of infection with A. lumbricoides among 445 school‐age children in Bangladesh at the first treatment and after two more rounds of treatment, each 6 months apart. The dotted line shows the trend which, when extrapolated, suggests only a small decline over two future rounds of treatment. Figure 10 also shows the mean worm burden at each round of treatment, which declines by almost 60% between the first and third treatment. If the linear decline was extrapolated it would lead to a substantial reduction in mean worm burden after five rounds of treatment. The relatively slow decline in worm burden achieved in the study in Bangladesh suggests that treatment would actually be best given every 3–4 months or three to four times a year rather than twice a year.
Figure 10.
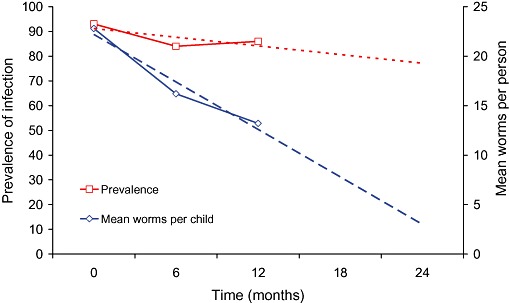
The prevalence of infection with Ascaris lumbricoides among 445 school‐age children in Bangladesh and the mean worm burden at baseline (0 months) and at two treatments 6 months apart (data from study of Hall et al. 1999). The dotted lines represent extrapolated trend lines if treatments had been given twice more at 6‐month intervals.
Figure 10 makes the point that the interval between treatments will influence the average intensity of reinfection that occurs, assuming a high efficacy, especially in the absence of any measures to prevent exposure to infectious stages in the environment or if only a proportion of the population is treated. Ideally the intervals between treatment should prevent the prevalence rising above 50%, the threshold at which the probability of moderate to heavy infections begins to increase exponentially (see Fig. 3).
The conclusion is that the frequency of treatment is therefore likely to affect the impact of treatment, and the aim should be to prevent moderate to large worm burdens from being reacquired. The consequence for this review is that it is difficult to compare studies of different numbers of treatments given over different periods. Nevertheless it can be useful to standardize gains in weight or any other parameter per unit of time, such as per year (Alderman et al. 2006). But, if reinfection is rapid, then two or even three treatments a year may have a greater impact than one, assuming that worms are the only constraint on growth.
3.4 Duration of follow‐up
It follows from the discussion in the previous section that, as well as the number of treatments given, the duration of follow‐up after treatment will affect the magnitude of any difference between a treated and an untreated control group, and therefore its statistical significance. This is illustrated in Fig. 11 which shows hypothetical data on the weight of two cohorts of undernourished children, 300 treated and 300 untreated, over a period of 3 years during which the treated group were kept almost free of worms. The children were 5 years old at the start of the study and were underweight, with an average body weight that was 2 SD below the National Center for Health Statistics median. If the periodic treatment of worms led to an extra gain in weight of 0.5 kg a year, or 3.5% of the initial mean weight, then it can be seen from the confidence intervals around the means in Fig. 11 that it is not until the third year that a statistically significant difference between groups is achieved.
Figure 11.
The average and 95% confidence intervals of body weight of two hypothetical groups of 300 children, one treated regularly with an anthelmintic and an untreated control group followed up for 3 years.
The duration of follow‐up is important because, as Section 2.4 has shown, a relatively small proportion of infected subjects will benefit from treatment so it is likely to take some time before the effects of repeated treatment are detectable in comparison with an untreated infected group. Some studies have attempted to measure a difference between groups in weight gain or growth over a very short period, such as 3 or 7 weeks after treatment (Hadju et al. 1996). Even though there was a statistically significant difference between study groups in such studies, it is too short a period to warrant inclusion in the review. Another study attempted to measure a change in haemoglobin and iron status over a period of 10 days after treatment (Karyadi et al. 1996), which is also a very short time in which to expect an improvement.
3.5 Outcomes measured and the need for controls
The primary outcomes usually measured during studies of the effect of anthelmintic treatment are changes in body weight, height, mid‐upper arm circumference and skinfold thickness. A change in haemoglobin concentration may also be expected if hookworm or T. trichiura occur. Measuring vitamin A status is hard to do as the concentration of retinol in serum is usually sustained from liver stores, so some sort of dose response test is usually used. Secondary outcome measures are anthropometric indices such as height‐for‐age and weight‐for‐age, which both require age to be known, ideally to within a month, and weight‐for‐height or body mass index. Indices of weight‐for‐height can only be calculated for girls younger than 10 years and boys younger than 11.5 years if the National Center for Health Statistics reference values are used (WHO 1983), which may limit their application in studies of school‐age children. Z‐scores of anthropometric indices are useful when controlling for the initial nutritional status of subjects based on the assumption that, when there is a large initial deficit, the impact of treatment is likely to be greater than if there is a small initial growth deficit. A change in z‐score can also help to give a perspective to the magnitude and nutritional significance of an improvement.
It cannot be assumed that a gain in body weight is due totally to an increase in lean body mass, because some increase could result from the deposition of fat in adipose tissue. Although there are now available weighing scales that can estimate the percentage body fat by applying standard equations to measurements of electrical impedance, they cannot usually be applied to young children, and may not apply to undernourished children in a developing country either.
Measurements of mid‐upper arm circumference and triceps skinfold thickness tend to be under‐used in studies of child growth after deworming. Their value lies in the ability to estimate the surface area of subcutaneous fat and muscle in the arm, assuming a standard area of bone (Gibson 1990) which could indicate both growth in lean tissue and the deposition of fat reserves.
A statistically significant weight gain can be achieved over a relatively short period, especially if food is available ad libitum, while a gain in height can take relatively longer. A period of about 12 weeks seems to be about the minimum period of follow‐up. A study of vitamin A and iron given to Tanzanian children after treatment with albendazole and praziquantel showed statistically significant extra gains in weight and height after 3 months of supplementation (Mwanri et al. 2000).
Studies of the effects of treating worms that cause blood loss require controls because the haemoglobin concentration can fluctuate naturally. Studies of giving iron supplements to school‐age children after deworming have shown statistical significance only because the mean value of the control group may go down in comparison with the treated children whose haemoglobin was sustained by the iron (Aguayo 2000; Hall et al. 2002; Roschnik et al. 2004). Natural fluctuations in haemoglobin may occur as a result of changes in the diet and diseases such as malaria, both or which may be seasonal.
3.6 Initial nutritional status
The impact of treatment on any indicator of nutritional status is likely to be related to the prior degree of undernutrition or deficiency, thus the margin for potential improvement. This was not the case in a study in South Africa of the effect of anthelmintic treatment and micronutrient‐fortified biscuits (Jinabhai et al. 2001b). One of the outcome measures was anthropometric status, but at the start of the study only 7.3% of children were stunted and 0.8% were underweight (Jinabhai et al. 2001b), not a large margin for improvement.
It may seem obvious, but it is also important that treatment and control groups should be similar before any treatment is given. A study of the impact of treatment with mebendazole given to young children as a part of an evaluation of the Integrated Management of Childhood Illness in western Kenya reported statistically significant differences in gains in weight, height and weight‐for‐age when infected and treated children were compared with infected and untreated controls (Garg et al. 2002). In this study the treated children were significantly more undernourished than the control group: the z‐score of weight‐for‐height of the group given the placebo was much greater than the treated children (treatment −0.89 versus control −0.19, P < 0.001) and there was a statistically significant difference in their initial weight‐for‐age as well (−1.43 versus −1.01, P < 0.001) (Garg et al. 2002). If the infected and treated children gained more weight it could easily have been because they started from a lower initial nutritional status and had a greater potential to achieve more catch‐up growth. Both the treatment and control groups need to be similar if the impact of treatment on growth or most nutritional variables is to be compared reliably.
3.7 Age of subjects
The age of subjects will influence the measurement of the impact of anthelmintic treatment. This is because growth is not linear during childhood and shows two spurts, the first during the first 2 years of life, and the second during adolescence. Although the prevalence and intensity of worm infections tends not to be as high during the first 2 years of life as during the school‐age years, the potential impact of worms early in life is greater, mainly because of the relative size of worms to their hosts, and the relative magnitude of any extra weight gain achieved after treatment. An extra weight gain of 500 g over a year by a 5‐year‐old girl whose weight is 2 SD below the mean is 3.6% of initial weight; for a 10‐year‐old it is 2.3% and for a 15‐year‐old it is 1.3%. Such an extra weight gain is of decreasing biological significance, unless it can be sustained each year, and will require a larger sample size to detect in the older age groups.
3.8 Remedial therapy after treatment
An implicit assumption of many randomized trials of anthelmintic treatment seems to be that removing worms will automatically lead to improvements in growth and nutritional status. This may happen, but only if the diet is adequate (Hall 2007). If worms have impaired growth, the haemoglobin concentration or micronutrient status, their effects are most likely to have been due to the mechanisms outlined in Section 1.3 which are worth repeating here: by feeding on the host's food, secretions, tissues and blood; by causing maldigestion or malabsorption; by effects on appetite and food intake; and by causing responses to infection that consume or divert resources unnecessarily. The losses or deficits caused by worms cannot be rectified or remedied simply by removing the worms alone, although halting any pathological processes is an important first step. After worms have been killed the need is for remedial treatment of the underlying nutritional deficits by providing energy, protein and micronutrients, so that catch‐up growth can be achieved. This is illustrated in Fig. 12 and discussed in some detail in Section 6.2.
Figure 12.
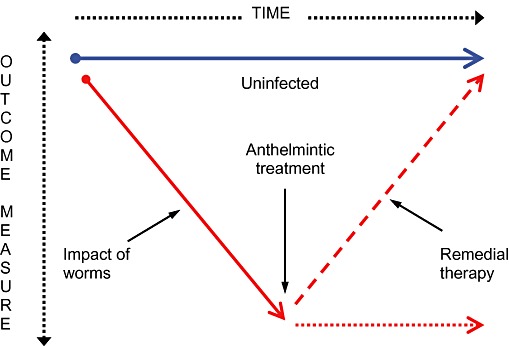
A conceptual model of the effect of worms on an outcome measure, and the need for remedial therapy after anthelmintic treatment to bring about a recovery.
Ideally, children should be protected from moderate to heavy infections throughout their childhood by repeated treatment to keep worm burdens low, by primary measures such as sanitation to prevent faeces containing worm eggs from getting into the environment, and by secondary measures to prevent exposure to worm eggs, such as personal hygiene.
3.9 Summary
There are a number of factors related to anthelmintic drugs and study design that need to be considered when either designing studies or evaluating papers that report studies.
-
•
Different drugs give a different cure rate and egg reduction rate for each species of intestinal nematode.
-
•
All anthelmintics are highly effective against A. lumbricoides.
-
•
Albendazole 400 mg and mebendazole 500 mg are the best treatments available for hookworms and T. trichiura.
-
•
At these dosages albendazole is more effective than mebendazole against hookworm infections, but both achieve egg reduction rates of 55–95%.
-
•
Neither albendazole nor mebendazole given as a single dose cures many infections with T. trichiura, but such treatment can reduce egg counts by 50%.
-
•
Albendazole and mebendazole may be a less effective treatment for T. trichiura in Asia than in Africa.
-
•
Three daily doses of albendazole or mebendazole may be needed to achieve high cure rates for T. trichiura.
-
•
Reinfection with worms can occur immediately after treatment, so treatment needs to be given periodically.
-
•
The interval between treatments may influence the impact on growth and nutritional status.
-
•
The subjects for study should be malnourished as well as infected.
-
•
As a minority of children (ranging from 10% to 40%) have moderate to heavy infections, not all will benefit to the same degree from treatment, and the impact on the average of any outcome measure may be diluted.
-
•
These factors should be taken into account so that the period of follow‐up after treatment should be sufficient to be able to detect a statistically significant effect.
-
•
It cannot be expected that nutritional status will improve and show catch‐up if the diet of subjects is inadequate to meet the extra requirements.
4. Aims and methods of the meta‐analysis
The aim of the review was to identify studies that had measured the effect of anthelmintic treatment given for infections with A. lumbricoides, T. trichiura and both species of hookworm on children's growth and weight gain, or on haemoglobin concentration.
4.1 Search terms
Table 9 shows the terms used in combination to search Medline. The search was limited to papers on humans in the following groups: infant, pre‐school, child and adolescent. Only papers in English were located. In addition, the reference list of each paper identified was scanned for other papers, so too was the Cochrane Review of 2000 (2000a, 2005) and the book by Stephenson (1987) Impact of Helminth Infections on Human Nutrition. One unpublished paper known to the principal author, who was a co‐investigator, was included (Partnership for Child Development 2001).
Table 9.
The terms used to search Medline for papers. MeSH denotes a specific Medical Subject Heading used as a search term
| Worms | Treatments | Outcomes |
|---|---|---|
| ‘intestinal worms’‘intestinal parasites’ geohelminth* helminth* | deworm* de‐worm* anthelmintic* antihelmintic* vermifuge | weight height length growth skinfold ‘arm circumference’‘nutritional status’ malnutrition anthropometry* |
| Ascaris roundworm trichuris whipworm necator Ancylostoma hookworm | benzimidazole albendazole mebendazole pyrantel levamisole piperazine | haemoglobin hemoglobin ‘iron status’ anaemia anemia |
| ‘Ascaris lumbricoides’[MeSH] Trichuris [MeSH]‘Necator americanus’[MeSH]‘Ancylostoma duodenale’[MeSH] | ‘Ascariasis/drug therapy’[MeSH]‘Ascariasis/prevention and control’[MeSH]‘Ascariasis/therapy’[MeSH]‘Hookworm infections/drug therapy’[MeSH]‘Hookworm infections/prevention and control’[MeSH]‘Hookworm infections/therapy’[MeSH] | retinol |
| ‘Trichuriasis/drug therapy’[MeSH]‘Trichuriasis/prevention and control’[MeSH]‘Trichuriasis/therapy’[MeSH] |
All papers identified were summarized under the following headings:
-
•
Design. A summary of the study design, including whether placebo controlled and blinded or not.
-
•
Follow‐up. The period children were studied after treatment, or the difference in time between the initial, interim and final measurements.
-
•
Location. The region and country where the study was carried out.
-
•
Age range.
-
•
Infection prevalence at baseline. The prevalence of each species of worm recorded.
-
•
Treatments. The drugs used and the doses given.
-
•
Sample size. The number of subjects studied in each group.
-
•
Outcomes. The principal outcome measures and any derived indices.
-
•
Findings. A summary of the major findings with standard deviations and the statistical significance of any differences between groups.
-
•
Notes. A summary of the prevalence of each intestinal nematode infection; a summary of the effect of treatment of worms; a summary of the degree of undernutrition estimated as z‐scores, the mean haemoglobin concentration or the prevalence of anaemia; and short notes on other major or different features of the study.
-
•
Included in Cochrane review of 2000 (recorded as Yes or No):
-
12
Dickson R, Awasthi S, Demellweek C, Williamson P. Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance (Review). Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No. CD000371. doi:10.1002/14651858.CD000371
-
•
Included in meta‐analysis. Either Yes, or the reason the study was excluded is given.
4.2 Inclusion criteria
Papers were included in the analysis if the following criteria were met:
-
•
Studies of treating children and adolescents (aged 1–19 years) for intestinal nematode worm infections with albendazole, levamisole, mebendazole, piperazine or pyrantel (pamoate or embonate).
-
•
An experimental group given the anthelmintic alone.
-
•
An untreated control group.
-
•
Random allocation to treatment and control groups.
-
•
An initial prevalence of infection in children in the study locality with any species of intestinal nematode worm of ≥50%, the threshold used by the WHO to classify a ‘high‐risk’ community (WHO 2006).
-
•
A duration of follow‐up of at least 12 weeks for all outcomes except the modified relative dose response test to an oral dose of retinol (Tanumihardjo et al. 1996a).
-
•
Outcomes measured included at least one primary outcome variable among weight, height, mid‐upper arm circumference, skinfold thickness, haemoglobin concentration, and a measure of serum retinol; or secondary outcomes based on weight‐for‐age, height‐for‐age or weight‐for‐height such as z‐scores or percentages of the median.
-
•
Data given as a mean with standard deviation and sample size, or enough data to calculate the standard deviation.
Data from the study of Koroma et al. (1996) were entered separately for urban and rural children.
4.3 Exclusion criteria
Papers were excluded from the meta‐analysis for one or more of the following reasons (the references cited are examples, and the list is not comprehensive):
-
•
Data on an outcome measurement not presented in a form that could be used, for example, change in percentage weight‐for‐height (Hadidjaja et al. 1998), difference in percentage improved (Kloetzel et al. 1982), change in percentage with anthropometric indices less than −2 z‐scores (Lai et al. 1995), weight gain presented as a graph (Sur et al. 2005), no data given for stated outcome measures for treated children (Olds et al. 1999); or data are unique so could not be combined with other studies (Tanumihardjo et al. 1996b).
-
•
No standard deviations of outcome measures given (Willett et al. 1979; Greenberg et al. 1981; Michaelsen 1985; Rousham & Mascie‐Taylor 1994; 1999a, 1999b; Fox et al. 2005) so not amenable to meta‐analysis using Review Manager 4.2 software.
-
•
The initial prevalence of worms was less than the ‘high risk’ threshold of 50% (Freij et al. 1979; Donnen et al. 1998; Awasthi et al. 2000; Awasthi & Pande 2001; Garg et al. 2002).
-
•
Inadequate control group or method of allocation, for example, infected subjects treated and compared with subjects who were lightly infected or uninfected (Callender et al. 1994; Mahendra Raj 1998; Mahendra Raj & Naing 1998; Bhargava et al. 2003); a sample size of two, one village where children were treated and another control village where they were not (Fernando et al. 1983); or no untreated controls (Forrester et al. 1998).
-
•
Treatment given for other conditions in addition to treatment for intestinal nematode worms, for example, metrifonate, which treats hookworm as well as Schistosoma spp. (1985, 1989a, 1989b); praziquantel to treat Schistosoma spp. combined with a drug to treat intestinal nematode worms (Kruger et al. 1996; Jalal et al. 1998; Beasley et al. 1999; Jinabhai et al. 2001a); or food or micronutrient supplements given in addition to anthelmintic treatment (Cerf et al. 1981; Marinho et al. 1991; Kruger et al. 1996; Palupi et al. 1997; Jinabhai et al. 2001b; Mwaniki et al. 2002).
-
•
Short period of follow‐up, for example, 3 and 7 weeks (Hadju et al. 1996), 10 days (Karyadi et al. 1996).
4.4 Meta‐analysis
Data from suitable papers were extracted and entered manually into RevMan version 4.2.8 for Windows. 2
Statistical comparisons were made between subjects treated with an anthelmintic drug and untreated controls for the outcomes listed in Table 13. For some studies the standard deviations had to be calculated from 95% confidence intervals and the sample size. For studies in which the mean and standard deviations of values before and after were given, the difference between mean values was used in the analysis with a pooled estimate of the standard deviation.
Table 13.
Summary of changes in primary and secondary outcome measures for studies in which albendazole or mebendazole were given to treat intestinal nematode worms
| No. of studies | No. of participants | Estimate (95% CI) | P | |
|---|---|---|---|---|
| Primary outcome measure | ||||
| Weight (kg) | 8 | 33 275 | 0.18 (0.13, 0.23) | <0.001 |
| Height (cm) | 6 | 5 227 | 0.08 (−0.01, 0.17) | 0.07 |
| MUAC (cm) | 6 | 3 228 | 0.30 (0.23, 0.37) | <0.001 |
| Triceps skinfold (mm) | 5 | 3 021 | 0.11 (0.03, 0.18) | 0.004 |
| Haemoglobin (g dL−1) | 5 | 2 178 | −0.93 (−2.97, 1.10) | 0.37 |
| % DR/R ratio | 2 | 204 | 0.17 (−0.60, 0.93) | 0.67 |
| Secondary outcome measure | ||||
| Weight‐for‐age z‐score | 4 | 468 | 0.06 (0.03, 0.08) | <0.001 |
| Height‐for‐age z‐score | 5 | 855 | 0.09 (0.06, 0.11) | <0.001 |
| Weight‐for‐height z‐score | 4 | 378 | 0.38 (0.30, 0.45) | <0.001 |
| Weight‐for‐age % median | 2 | 242 | 3.16 (2.51, 3.81) | <0.001 |
| Height‐for‐age % median | 2 | 242 | 0.31 (0.14, 0.47) | <0.001 |
| Weight‐for‐height % median | 2 | 242 | 2.73 (2.03, 3.44) | <0.001 |
CI, confidence intervals; MUAC, mid‐upper arm circumference; DR/R, dehydroretinol/retinol.
The weighted mean difference between treatment and control groups was calculated with 95% confidence intervals in RevMan 4.2 software (RevMan 2003) for each variable using a fixed effects model. Studies were disaggregated into subcategories by treatment except that studies in which mebendazole and albendazole were given were combined, as the two drugs are chemically similar and have the same mode of action.
5. Results of the meta‐analysis
A total of 58 published papers were found, summaries of which can be found in the Appendix. They were not all unique studies as some data were reported in more than one paper. A total of 40 papers were not included in the analysis because they either met exclusion criteria or did not meet inclusion criteria; this left 18 studies for analysis. One additional unpublished study, a randomized cluster trial of albendazole in Vietnamese school children, was included (Partnership for Child Development 2001), increasing the sample to 19 papers, as follows:
Adams et al. (1994). Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. Journal of Nutrition 124, 1199–1206.
Alderman et al. (2006) Effect on weight gain of routinely giving albendazole to preschool children during child health days in Uganda: cluster randomized controlled trial. British Medical Journal 333, 122–124.
Dossa et al. (2001). Impact of iron supplementation and deworming on growth performance in preschool Beninese children. European Journal of Clinical Nutrition 55, 223–228.
Gupta & Urrutia (1982). Effect of periodic antiascaris and antigiardia treatment on nutritional status of preschool children. American Journal of Clinical Nutrition 36, 79–86.
Koroma et al. (1996). Effects of albendazole on growth of primary school children and the prevalence and intensity of soil‐transmitted helminths in Sierra Leone. Journal of Topical Pediatrics 42, 371–372.
Northrop‐Clewes et al. (2001). Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. American Journal of Clinical Nutrition 73, 53–60.
Partnership for Child Development (2002, unpublished). A randomised cluster trial of six‐monthly deworming and its effects on the growth and educational achievements of Vietnamese school children.
Sarker et al. (2002). Effect of deworming on nutritional status of Ascaris infested slum children of Dhaka, Bangladesh. Indian Pediatrics 39, 1021–1026.
Simeon et al. (1995). Treatment of Trichuris trichiura infections improves growth, spelling scores and school attendance in some children. Journal of Nutrition 125, 1875–1883.
Stephenson et al. (1989c). Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura and Ascaris lumbricoides infections. American Journal of Tropical Medicine and Hygiene 41, 78–87.
Stephenson et al. (1993a). Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once‐ or twice‐yearly treatment with albendazole. Journal of Nutrition 123, 656–665.
Stephenson et al. (1993b). Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. Journal of Nutrition 123, 1036–1046.
Stoltzfus et al. (1997). School‐based deworming program yields small improvement in growth of Zanzibari school children after one year. Journal of Nutrition 127, 2187–2193.
Stoltzfus et al. (1998). Effects of the Zanzibar school‐based deworming program on iron status of children. American Journal of Clinical Nutrition 127, 179–186.
Stoltzfus et al. (2004). Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelmintic treatment improves growth, appetite and anemia in Zanzibari preschool children. Journal of Nutrition 134, 348–356.
Tanumihardjo et al. (1996b) Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. Journal of Nutrition 126, 451–457.
Tanumihardjo & Permaesih (2004). Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming treatments. European Journal of Clinical Nutrition 58, 1223–1230.
Thein‐Hlaing et al. (1991). A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 523–528.
Watkins & Pollitt (1996). Effect of removing Ascaris on the growth of Guatemalan schoolchildren. Pediatrics 97, 871–876.
Table 10 shows a summary of the key features of the 19 studies. The studies could not be disaggregated by age into pre‐school (1–5 years) and school age (6–15 years) because several had overlapping age ranges, particularly the largest study, undertaken by Alderman et al. (2006) in Uganda.
Table 10.
A summary of main characteristics of the studies included in the meta‐analysis
| Authors | Ascaris (%) | Trichuris (%) | Hookworm (%) | Any | Age range | n | Drug | Dose | Frequency | Treatment | Control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams et al. (1994) | 29 | 84 | 93 | NR | 5–10 y | 56 | ABZ | 400 mg d−1 × 3 d | Once | None | Placebo |
| Alderman et al. (2006) | ∼18 | ∼7 | ∼44 | ∼56% | 1–7 y | 27 995 | ABZ | 400 mg | 2–5 times every 6 mo | None | Untreated |
| Dossa et al. (2001) | 38 | 47 | 13 | NR | 3–5 y | 177 | ABZ | 200 mg d−1 × 3 d | Twice, 1 mo apart | Iron | Placebo |
| Gupta & Urrutia (1982) | 51–72 | 9 | 0 | NR | 24–61 mo | 159 | PPZ | 75 mg kg−1 d−1 × 2d | Once | None | Placebo |
| Koroma et al. (1996). Rural | 46 | 25 | 1 | NR | 6–10 y | 98 | ABZ | 400 mg | Once | None | Placebo |
| Koroma et al. (1996). Urban | 32 | 10 | 65 | NR | 6–10 y | 99 | ABZ | 400 mg | Once | None | Placebo |
| Northrop‐Clewes et al. (2001) | 78 | 65 | 4 | NR | 2–5 y | 117 | PP | 10 mg kg−1 | Single dose then | None | Placebo |
| MBZ | 500 mg | Bimonthly for 1 year | |||||||||
| PCD (2002, unpublished) | 71 | 84 | 7 | 93% | 7–8 y | 2 659 | ABZ | 400 mg | Every 6 mo, 5 times | None | Untreated |
| Sarker et al. (2002) | 78 | NR | NR | NR | 2–12 y | 85 | PP | 11 mg kg−1 | Once | None | Placebo |
| Simeon et al. (1995) | 42–50 | 100 | NR | NR | 6–12 y | 407 | ABZ | 400 mg d−1 × 2 d | Once | None | Placebo |
| Stephenson et al. (1989c) | 49 | 97 | 87 | NR | 6–16 y | 150 | ABZ | 400 mg | Once | None | Placebo |
| Stephenson et al. (1993a) | 26–35 | 81–92 | 85–88 | NR | 6–15 y | 284 | ABZ | 600 mg | Once or twice 3.6 m later | None | Placebo |
| Stephenson et al. (1993b) | 41 | 98 | 96 | NR | 7–13 y | 53 | ABZ | 600 mg | Once | Food | Placebo |
| Stoltzfus et al. (1997) | 66–76 | 95–97 | 91–96 | NR | Not stated | 3 063 | MBZ | 500 mg | 2× or 3× per year | None | Unclear |
| Stoltzfus et al. (1998) | 67–76 | 95–97 | 91–96 | NR | Not stated | 2 924 | MBZ | 500 mg | 2× or 3× per year | Iron | Untreated |
| Stoltzfus et al. (2004) | 42–44 | 71–72 | 54 | NR | 6–71 mo | 459 | MBZ | 500 mg | Once every 3 mo for 1 y | Iron | Placebo |
| Tanumihardjo et al. (1996a) | 100 | 17–27 | NR | NR | 0.6–6.6 y | 309 | ABZ | 400 mg | Once | Retinol | Placebo |
| Tanumihardjo & Permaesih (2004) | 100 | 28 | NR | NR | Not stated | 51 | ABZ | 400 mg | Once | Retinol | Untreated |
| Thein‐Hlaing et al. (1991) | 81–83 | 5–7 | 1–2 | NR | 2–12 y | 1 206 | LEV | Not stated | Once | None | Untreated |
| Watkins & Pollitt (1996) | 91–92 | 78–85 | NR | NR | <12 y | 228 | ABZ | 400 mg | Once | None | Placebo |
NR, not reported; ABZ, albendazole; LEV, levamisole; MBZ, mebendazole; PCD, Partnership for Child Development; PP, pyrantel pamoate; PPZ, piperazine; d, days; mo, months; y, years.
5.1 Geographic origin of studies
Table 11 shows the countries represented in the papers found and used in this review. Of all 58 papers found, nearly 50% reported data from Africa. Of the 19 papers used in the analysis, a little more than 50% reported data from Africa.
Table 11.
The countries from which data were published in papers on the effect of anthelmintic treatment on the growth and nutritional status of children. Also shown is the number of times each country is represented in papers found during the literature search and the papers eventually used in the meta‐analysis. One paper reported data from China, the Philippines and Kenya (Olds et al. 1999) so the total number of papers is 58 rather than 60. All other papers only presented data from one country
| Country | Papers found | Papers used |
|---|---|---|
| Benin | 1 | 1 |
| Botswana | 1 | 0 |
| Ethiopia | 2 | 0 |
| Kenya | 11 | 4 |
| Sierra Leone | 1 | 1 |
| South Africa | 4 | 0 |
| Tanzania | 6 | 3 |
| Uganda | 1 | 1 |
| Zaire | 1 | 0 |
| China | 1 | 0 |
| Indonesia | 7 | 2 |
| Malaysia | 3 | 0 |
| Myanmar | 1 | 1 |
| Philippines | 1 | 0 |
| Vietnam | 1 | 1 |
| Bangladesh | 5 | 2 |
| India | 3 | 0 |
| Sri Lanka | 1 | 0 |
| Brazil | 2 | 0 |
| Guatemala | 2 | 2 |
| Haiti | 2 | 0 |
| Jamaica | 2 | 1 |
| Mexico | 1 | 0 |
| Total | 58 | 19 |
5.2 Estimates of effects
Table 12 presents summary data on the impact of treatment on 12 variables for all studies in which intestinal worms were treated with any effective anthelmintic. The drugs used were albendazole and mebendazole, levamisole, piperazine or pyrantel pamoate. There were statistically significant differences for most variables related to growth; but the outcomes for micronutrients were not statistically significant.
Table 12.
Summary of changes in primary and secondary outcome measures for studies in which any drug to treat intestinal nematode worms was given. The drugs were: albendazole and mebendazole, levamisole, piperazine or pyrantel pamoate
| No. of studies | No. of participants | Estimate (95% CI) | P | |
|---|---|---|---|---|
| Primary outcome measure | ||||
| Weight (kg) | 11 | 33 860 | 0.21 (0.17, 0.26) | <0.001 |
| Height (cm) | 9 | 5 801 | 0.11 (0.03, 0.19) | 0.01 |
| MUAC (cm) | 7 | 3 325 | 0.30 (0.23, 0.37) | <0.001 |
| Triceps skinfold (mm) | 5 | 3 021 | 0.11 (0.03, 0.18) | 0.04 |
| Haemoglobin (g dL−1) | 5 | 2 178 | −0.93 (−2.97, 1.10) | 0.37 |
| % DR/R ratio | 2 | 204 | 0.17 (−0.60, 0.93) | 0.67 |
| Secondary outcome measures | ||||
| Weight‐for‐age z‐score | 5 | 557 | 0.06 (0.03, 0.08) | <0.001 |
| Height‐for‐age z‐score | 6 | 961 | 0.09 (0.06, 0.11) | <0.001 |
| Weight‐for‐height z‐score | 4 | 378 | 0.38 (0.30, 0.46) | <0.001 |
| Weight‐for‐age % median | 4 | 401 | 2.52 (1.95, 3.08) | <0.001 |
| Height‐for‐age % median | 4 | 401 | 0.27 (0.12, 0.42) | <0.001 |
| Weight‐for‐height % median | 3 | 323 | 2.64 (1.97, 3.30) | <0.001 |
CI, confidence intervals; MUAC, mid‐upper arm circumference; DR/R, dehydroretinol/retinol.
Table 13 presents summary data on the impact of treatment on 12 variables for all studies in which intestinal worms were treated with albendazole or mebendazole only, as these drugs are now the most widely used and most broadly effective anthelmintics.
5.3 The figures and how to interpret them
13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 show the mean differences between treated and control groups for each study grouped vertically by the drugs used. The mean is shown as a box around the vertical line of zero difference, with 95% confidence intervals shown as bars. The diamonds represent the weighted mean difference between the treated and control groups for each treatment group and in total. The percentage weight given to each study, as well as the numerical value of the estimated difference with 95% confidence intervals, is shown. It should be noted that the scale in all figures except for 22, 25 and 25 is from −4 (favours control) to 4 (favours treatment).
Figure 13.
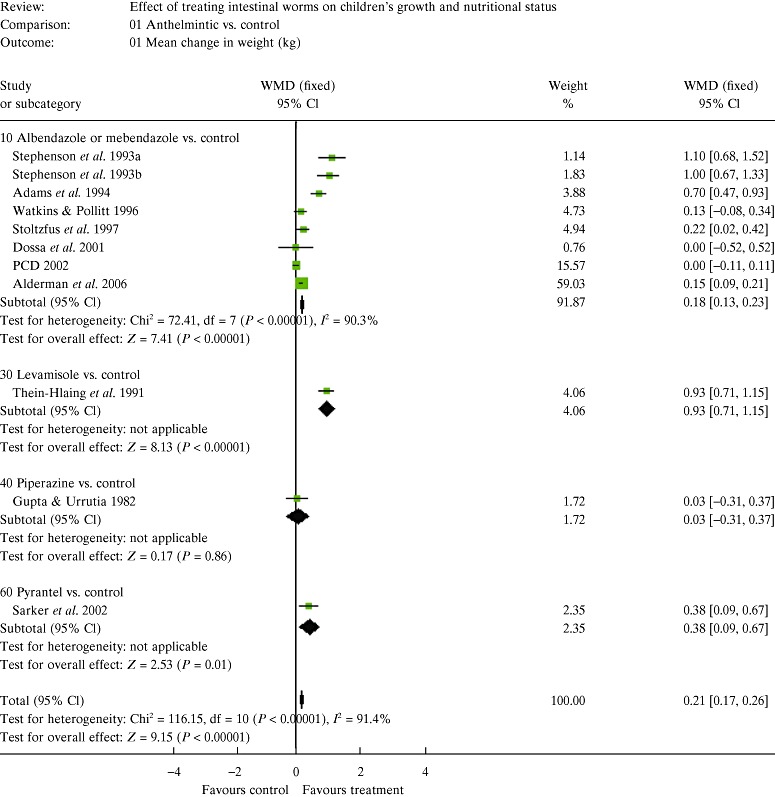
The effects of treating intestinal worms on body weight (kg). To interpret the figure, see Section 5.3. CI, confidence intervals; PCD, Partnership for Child Development; WMD, weighted mean difference.
Figure 14.
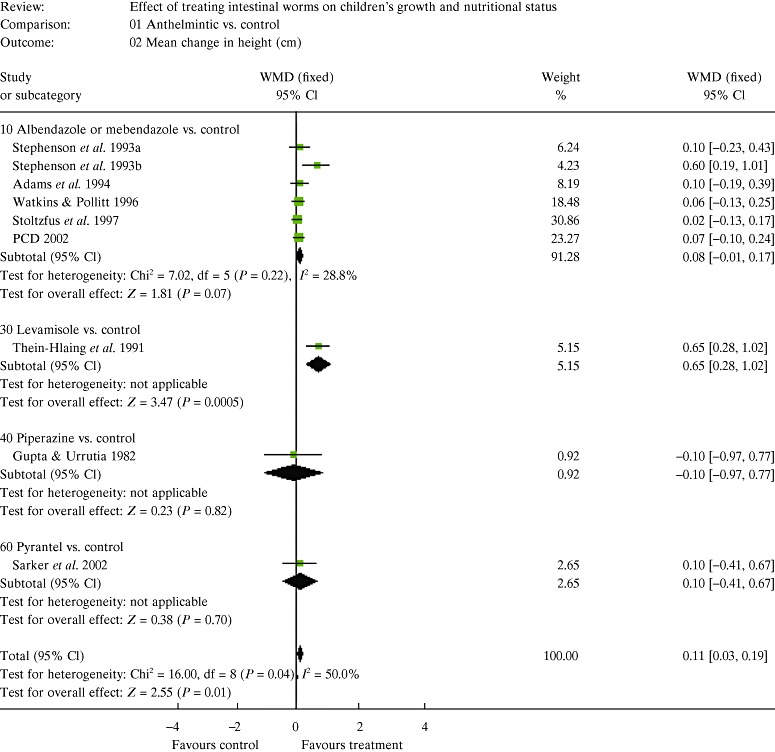
The effects of treating intestinal worms on height (cm). To interpret the figure, see Section 5.3. CI, confidence intervals; PCD, Partnership for Child Development; WMD, weighted mean difference.
Figure 15.
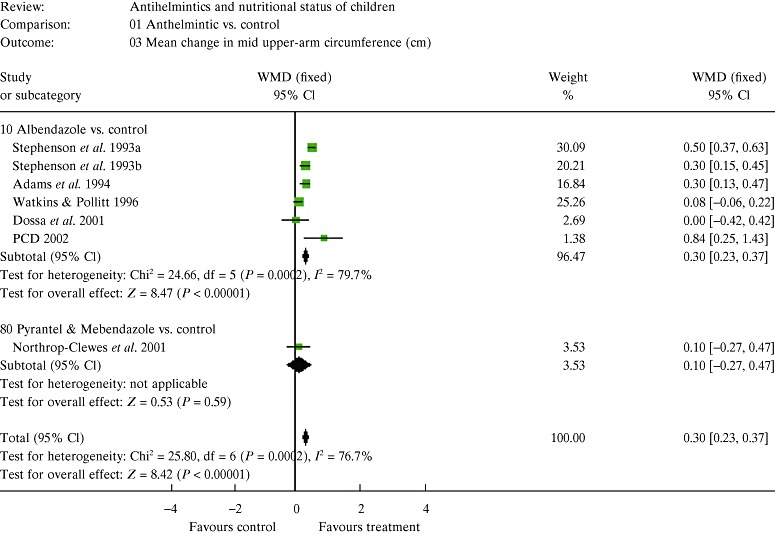
The effects of treating intestinal worms on mid‐upper arm circumference (mm). To interpret the figure, see Section 5.3. CI, confidence intervals; PCD, Partnership for Child Development; WMD, weighted mean difference.
Figure 16.
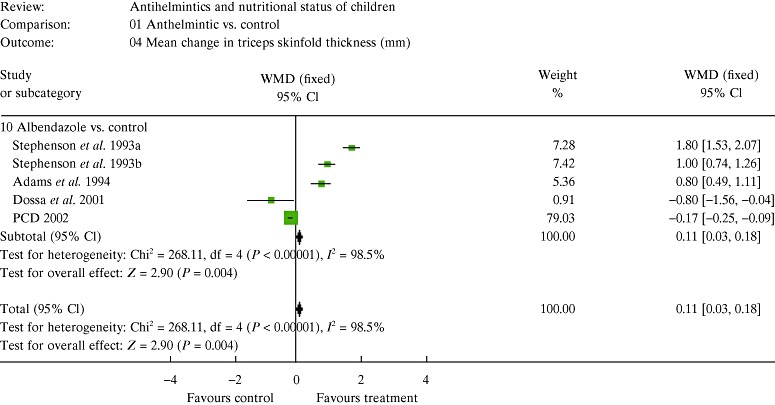
The effects of treating intestinal worms on triceps skinfold thickness (mm). To interpret the figure, see Section 5.3. CI, confidence intervals; PCD, Partnership for Child Development; WMD, weighted mean difference.
Figure 17.
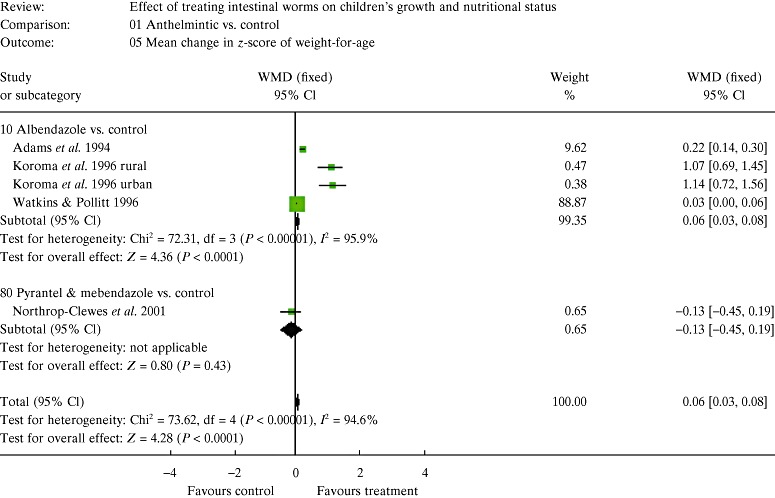
The effects of treating intestinal worms on z‐score of weight‐for‐age. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 18.
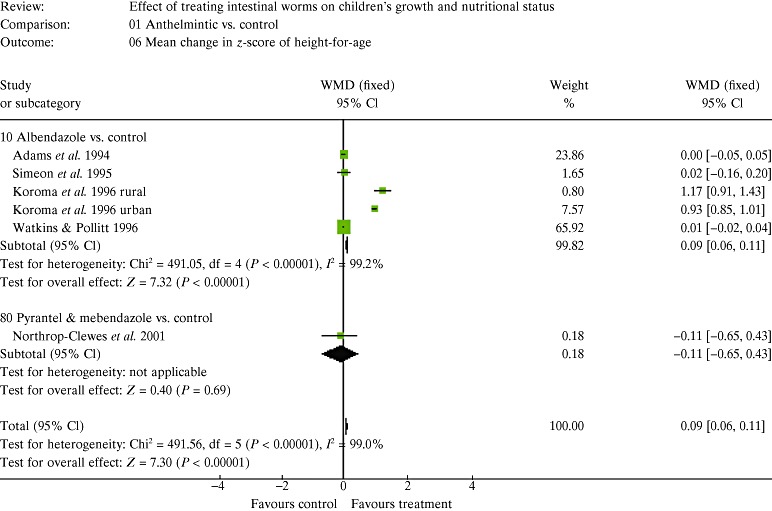
The effects of treating intestinal worms on z‐score of height‐for‐age. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 19.
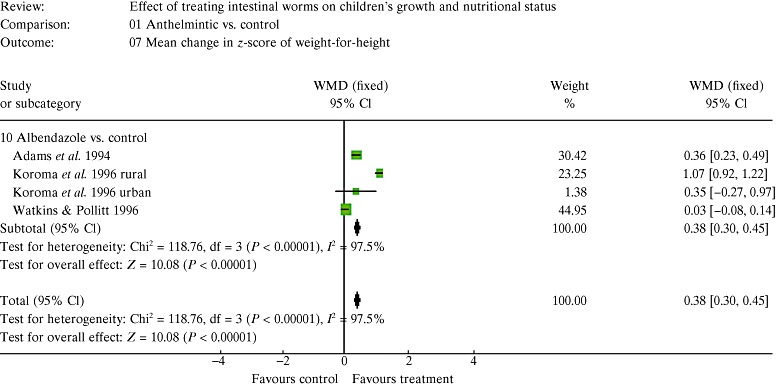
The effects of treating intestinal worms on the difference in z‐score of weight‐for‐height. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 20.
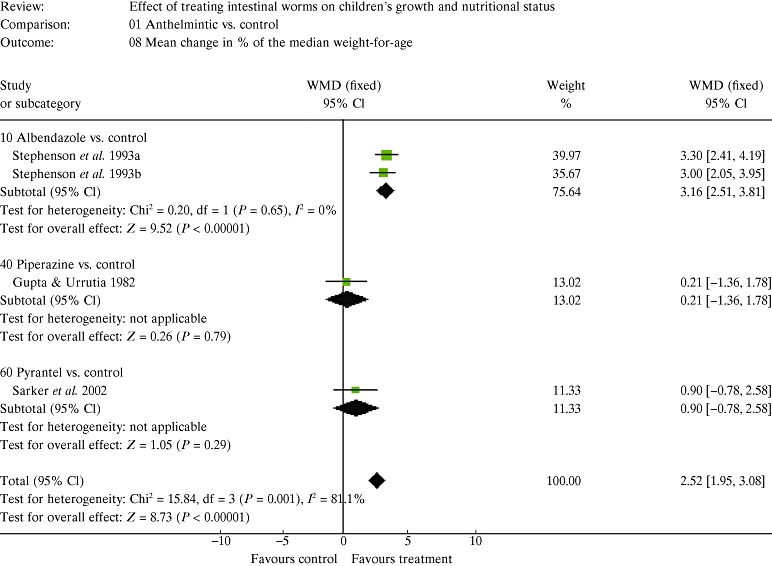
The effects of treating intestinal worms on percentage of the median weight‐for‐age. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 21.
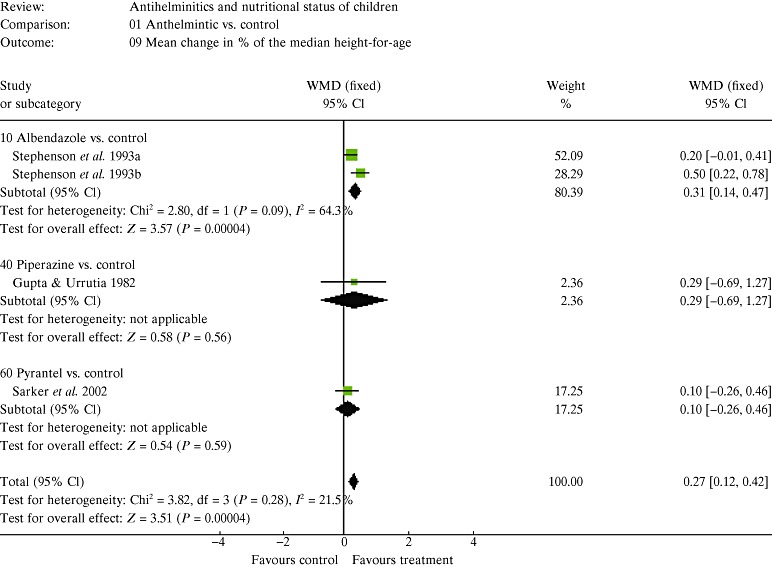
The effects of treating intestinal worms on percentage of the median height‐for‐age. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 22.
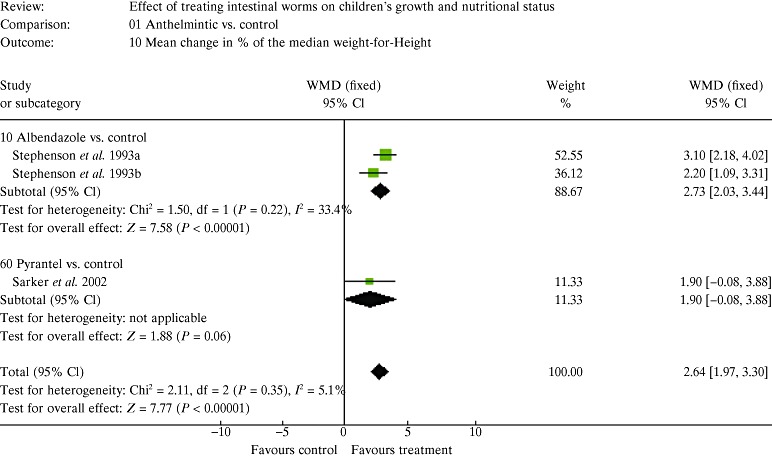
The effects of treating intestinal worms on percentage of the median weight‐for‐height. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 23.
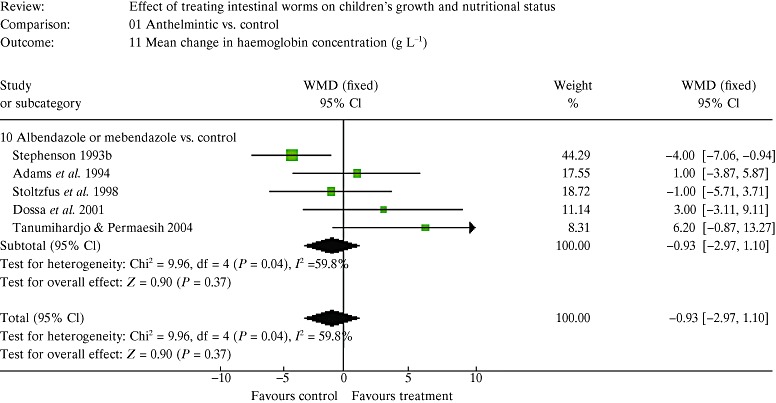
The effects of treating intestinal worms on haemoglobin concentration (g dL−1). To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 24.
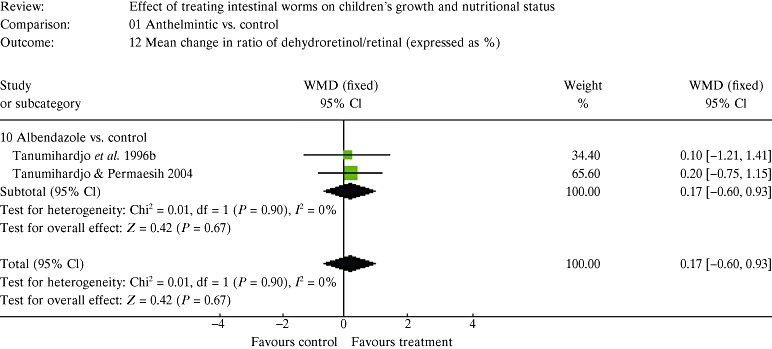
The effects of treating intestinal worms on the dehydroretinol/retinol ratio expressed as a percentage. To interpret the figure, see Section 5.3. CI, confidence intervals; WMD, weighted mean difference.
Figure 25.
A 2 × 2 factorial design to estimate the effect of deworming with or without a nutritional treatment or not.
5.4 Sources of error or bias
The decision to exclude studies in which the initial prevalence of infection is <50% is somewhat arbitrary, but is based on the WHO threshold which classifies them as ‘low‐risk’ communities (WHO 2006). Ideally all children taking part in studies of anthelmintic treatment would be infected with at least one species of worm, just as any drug trial would be conducted only on infected individuals. But most trials of the impact of anthelmintics on child growth tend to study groups of children rather than infected subjects only, so tend to be trials of effectiveness rather than efficacy. This type of study is driven also because of ethical problems with having infected but untreated children as controls, so studies tend to be carried out in communities in which the prevalence is known, and whether individual subjects are infected or not is usually unknown. Applying a threshold prevalence of 50% or more means that a half of all subjects or fewer will not benefit from anthelmintic treatment, and their inclusion in the study sample will to some degree dilute the impact of treatment on the infected majority. The threshold prevalence of 50% does not serve to maximize the impact of treatment, a prevalence of 100% is needed to do that, but a prevalence higher than 50% serves to reduce the effect of uninfected subjects. This means that the conclusions of the meta‐analysis can only be applied to circumstances in which the prevalence is more than 50% and excludes what the WHO calls ‘low‐risk’ communities, in which the prevalence of infection with any species of worm lies between 20% and 49% (WHO 2006).
About a half of all papers found and used in the analysis reported data from Africa. All of the papers reporting African data were from sub‐Saharan Africa, reflecting concern for the effects of worms in African children.
In many parts of sub‐Saharan Africa children are concurrently infected with species of Schistosoma, which may cause loss of appetite, internal bleeding, and tissue damage and inflammation resulting from reactions to the eggs of worms when they become lodged in tissues. Studies were excluded from this analysis in which subjects were also treated with praziquantel or metrifonate, the drugs most commonly used to treat Schistosoma spp., as this would overestimate the effect of treating intestinal nematodes. This could, however, have led to a bias against studies from sub‐Saharan Africa. But this did not seem to be the case, as a larger proportion of all papers from Africa were included in the final analysis than in Africa, Asia or the Indian subcontinent. Of the papers found and the papers used, the proportion from Africa was similar in both categories (28/59 or 47% and 11/24 or 46%).
6. Discussion
This meta‐analysis indicates that if the prevalence of intestinal nematodes is 50% or more then giving anthelmintic drugs leads to significant extra gains in weight, height, mid‐upper arm circumference and skinfold thickness in comparison with untreated controls. Table 12 shows the weighted average changes recorded in the studies found. The gains in weight and height also led to improvements in indices of anthropometric status in studies in which only these outcomes were reported. There was no evidence of a significant effect of treating intestinal nematode infections on haemoglobin concentration. Only two studies, both by the same author in Indonesia, were reported of the dehydroretinol to retinol ratio, and found no difference between treated and control groups (Tanumihardjo et al. 1996b; Tanumihardjo & Permaesih 2004).
6.1 Magnitude of effects
It is not possible to say anything conclusive about the absolute magnitude of any effects of giving treatment, for a number of reasons. The studies summarized were different in terms of:
-
•
the species and mixture of species of worms;
-
•
the distribution of worms between hosts;
-
•
the drug and dose used, leading to variability in the efficacy of each treatment;
-
•
the number of treatments given and intervals between them; and
-
•
the initial degree of undernutrition, the age and current health of subjects;
-
•
the period of follow‐up after treatment, or the gap between the initial and final measurements.
The magnitude of the extra increases shown in 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 are not standardized as gains per unit time, which would have enabled them to be better compared, and would have put any improvement into some sort of perspective. A extra gain in weight of 0.2 kg in 4 months is much bigger and more significant in terms of growth than a extra gain of 0.2 kg over a year. Any gain per unit time will also be influenced by the frequency of treatment, thereby keeping worm burdens down, perhaps. This was indicated in the study in Uganda: children who were dewormed at least every 6 months gained an extra 10% in weight compared with the 5% extra by children who were treated only annually (Alderman et al. 2006). However, this could have been confounded by the fact that other health services were delivered at the Parish Health Fairs to children who came more often, or perhaps the regular attendance of children at Health Fairs also reflected better care at home.
If the magnitude of any gain is related to the initial degree of undernutrition, then it might be better to standardize the gain per unit time as a proportion of the initial value, although this may be statistically difficult to do. This could be important for the haemoglobin concentration. For example, an increase of 10 g L−1 when the initial average was 90 g L−1 (11%) is more significant than an increase of 10 g L−1 when the initial average was 110 g L−1 (9%).
Nothing can be said about whether any particular drug is more effective than another at bringing about an improvement in a nutritional outcome, although egg reduction rate is likely to be a good indicator of this. The benzimidazole drugs are currently the most efficacious treatments available for treating intestinal nematode worms. Albendazole was the most commonly used drug (Table 10), and is effective to differing degrees against the three main species of worms, as Table 7 shows. Mebendazole has a similar efficacy but was given less often, probably because it used to be given as a dose of 100 mg a day for 3 days rather than as a single dose of 500 mg, as it is now used. A single dose of an effective drug is a crucially important attribute for a helminth disease control programme. However, neither albendazole nor mebendazole is highly effective against T. trichiura (Bennett 2000), and a failure to achieve a good egg reduction rate could contribute to a smaller impact of treatment than could be achieved by giving multiple doses.
6.2 Treatment alone is not enough
Whatever extra gains in nutritional or anthropometric outcomes are achieved after treatment with an anthelmintic drug, they do not occur solely as a result of that treatment. Catch‐up growth, for example, requires extra nutrients and energy, and if they are not available after treatment then growth rates are likely to remain unchanged. Alternatively a statistically significant difference between a treatment and control group could occur during a study because an untreated control group remain persistently diseased as a result of their infections, and thus gain less weight than the treated group.
The lack of extra food to meet the needs for growth could explain the lack of impact of deworming in many studies, such as the large cluster trial in Vietnam (Partnership for Child Development 2001). The expulsion of worms from the gut may remove a constraint that acts through effects on appetite and food intake, digestion and absorption, or because of the diversion of nutrients in response to infection. To achieve the maximum growth rate after treatment, energy, protein and micronutrients need to be provided to children, preferably ad libitum. Most studies did not give food supplements after treatment, but one that did showed an improved appetite measured as food intake as well as improved growth (Stephenson et al. 1993a).
Catch‐up growth is possible (Golden 1994) especially if the deficits occur early in childhood and are treated adequately. Studies of children treated for Trichuris dysentery syndrome, admittedly an acute disease, have shown an average gain of nearly 11 cm in height and 4 kg in weight a year, which was more than 2 SD above the expected gain in height and weight of British children of the same age (Cooper et al. 1995). Some of the 5‐year‐old children studied showed an annual growth velocity of nearly 20 cm a year and a weight gain of 10 kg.
To achieve such catch‐up growth children need to be fed ad libitum, not just with energy and protein, but also with micronutrients, so that no nutrient is limiting and the maximum possible growth is achieved (Hall 2007). If studies of deworming have failed to show an impact on growth or weight gain, it could be because children were not getting enough of the food and micronutrients they needed to achieve catch‐up growth. And the studies that have shown a statistically significant impact of treatment may have underestimated the potential effect if children did not have an ideal and balanced diet given ad libitum. No published studies have been found of randomized controlled trials of deworming with or without any form of remedial feeding given ad libitum; most studies have only given supplementary food or supplementary micronutrients.
A problem with giving food supplements is that there can be substitution, in which people eat less food at home after being fed at school or work (Wolgemuth et al. 1982). Or supplementary food may benefit the better off most: a study of a school nutrition programme in Vietnam found that better nourished children gained more weight than undernourished children (Hall et al. 2007), which is not what is hoped for in school feeding programmes.
The need for adequate remedial treatment is important when the outcome being measured is the haemoglobin concentration. A study in Tanzania of giving iron supplements to anaemic children after treating their infections with hookworm and S. haematobium showed no improvement in the mean haemoglobin concentration, but the serum ferritin concentration increased, an indicator of improved iron status (Beasley et al. 2000). This suggests that iron was not the micronutrient limiting the haemoglobin concentration.
This does not mean that improvements in growth and nutritional status cannot be achieved by deworming alone, especially if there are improvements in appetite and food is available to children. To have the maximum effect on growth and nutritional status, therapeutic nutrients are needed as well. A failure to have an impact could be because of this lack, or because the worms were not an important cause of any nutritional deficit in the first place.
The costs of only giving supplementary food can be quite high: the World Food Programme estimate that it costs $34 a year to feed a child in school (World Food Programme 2006). An alternative is to provide micronutrient supplements, a deficiency of which may also act to impair growth, and which have been shown to reduce the intensity of reinfection with schistosomes (Olsen et al. 2003). A multiple micronutrient supplement is a much less expensive intervention than food, and may cost only around one US cent per child per day if purchased in large quantities. But the key point is that if any nutrient is not provided in sufficient amounts to meet demands, and becomes rate limiting, then growth after deworming may be constrained.
The weighted average gains in parameters of growth shown in Table 12 therefore do not indicate what extra growth might be achieved after treatment or indicate the potential for growth, they only estimate what can be achieved after giving treatment. If a study included in the analysis showed no statistically significant difference between the treatment and control groups, the question is: did this happen because there was no effect, or because treated children were lacking sufficient energy or nutrients to show extra growth and weight gain? A lack of effect in such studies will serve to reduce the weighted average, and so underestimate the potential impact of treatment. It is also entirely possible that anthelmintic treatment may have had no effect even if children had been adequately nourished. But, as there are plausible biological mechanisms by which moderate to heavy burdens of worms can affect nutritional status, a beneficial impact on some (but not all) children seems likely.
This touches upon the aggregated distribution of worms and the uneven distribution of the impact of treatment. If disease and malnutrition are a consequence of moderate to heavy infections, then in any given sample of children a minority will benefit most from treatment, so the effect of treatment will not be evenly distributed between children. For example, if 25–49 A. lumbricoides is arbitrarily considered to be a moderate infection in children and 50 or more worms is a heavy infection, then of the 1069 children aged 1–14 years studied in an urban slum in Bangladesh, 18% were moderately heavily infected and 7% heavily infected (data from Hall et al. 1999). If only these children benefited from treatment then the impact on this 25% would in effect be diluted in the lack of change in the remaining 75%. The average treatment effect therefore includes a large proportion of children who would not be expected to benefit, and would underestimate the potential impact on moderately to heavily infected children. The proportion of lightly infected children will be very large when the prevalence is low, and when it is less than 50% it might be expected that very few children would benefit substantially from treatment. The general consequence is that the study average will not capture the larger benefit experienced by any moderately to heavily infected children.
One way to control for this would be to analyse the relationship between any outcome and an estimate of the intensity of infection, such as the concentration of eggs in faeces. This method was used by Stephenson et al. (1993a) in their studies of the effect of anthelmintic treatment on appetite. This can only be done if a prior individual diagnosis is made and, if it is, it could be considered to be unethical to leave children untreated whose infections have been diagnosed. One way to deal with this dilemma is to randomly allocate subjects to treatment and control groups but only diagnose infections in the subjects who will be treated. This was done in a study in Tanzania in which infections were diagnosed in the control group at the end of the study (Bhargava et al. 2003). The problem with doing this is that the intensity of infections can change in the control group as worms are lost or gained during a long study, so the treatment and control groups cannot be compared at any point during the study.
Two assumptions are commonly made about the effect of the intensity of infection. First, that treatment has an impact only above a threshold worm burden, which seems reasonable, although the threshold number is likely to be dependent on factors such as the size and current health of the host. Second, that above the threshold the impact of treatment on more heavily infected individuals is linear. This may not be the case, but is very difficult to assess without having heavily infected children as controls during a study, something that would be unethical. An alternative is to examine the nature of the relationship between the intensity of infection with a worm and an outcome that is easily measurable, such as hookworm and haemoglobin concentration. Cross‐sectional studies typically find a non‐linear relationship so that the subjects with the heaviest infections have a markedly lower haemoglobin concentration than lightly infected individuals (Roche & Layrisse 1966; Srinivasan et al. 1987; Pritchard et al. 1991; Lwambo et al. 1992).
This meta‐analysis found no statistically significant effect of treating intestinal nematode worms on the mean haemoglobin concentration of children (8, 9). Yet the potential impact of treating worms that cause internal bleeding, dysentery and the loss of iron is large. In Africa in particular, many children are infected with species of Schistosoma in addition to hookworms, which makes it difficult to assess the impact of treating the intestinal nematodes alone. An important study in Tanzania showed that supplements of vitamin A and iron are needed if rapid improvements in haemoglobin concentration are to be achieved after deworming (Mwanri et al. 2000). All children in the study were given albendazole and praziquantel, then were divided into four groups to receive on 3 school days a week either iron and vitamin A placebo, vitamin A and an iron placebo, iron plus vitamin A, or iron and vitamin A placebos (Mwanri et al. 2000). The haemoglobin concentration of children given both placebos rose by an average of only 3.6 g L−1 in the 12 weeks after treatment which might have been a statistically significant increase, but there was no untreated control group to allow this to be assessed. The effects of the supplements of iron and vitamin A were partially additive and achieved an increase in haemoglobin concentration of 22 g L−1 over the same period compared with 13.5 g L−1 for vitamin A alone and 17.5 g L−1 for iron alone. This study illustrates the importance of giving micronutrient supplements after treatment in order to achieve a rapid increase in haemoglobin concentration and may explain why no statistically significant effect of anthelmintic treatment alone was detected in the meta‐analysis. If the change of 3.6 g L−1 over 12 weeks in the control group reflects the effect of anthelmintic treatment alone, then it might have taken six times as long to achieve an increase of 22 g L−1, or nearly a year and a half. The inclusion of vitamin A in addition to iron makes the important point that not all anaemia is iron deficiency anaemia, and this could explain the results of a study in Tanzania that gave iron alone and failed to achieve a statistically significant increase in haemoglobin concentration (Beasley et al. 2000).
6.3 The Cochrane Collaboration Review
The Cochrane Collaboration supported a meta‐analysis in 2000 (2000a, 2000b). The reviewers noted heterogeneity between the results of the trials they included, and considered that they could be due to a number of factors. These were: the prevalence and intensity of infection, so that only heavily infected children benefited from treatment; the site of the study (school, community or health facility); the age of children; the prior nutritional status of children; and the manufacturer of the drug. The reviewers concluded that ‘there is some limited evidence that routine treatment of children in areas where helminths are common has effects on weight gain, but this is not consistent between trials’. The heterogeneity noted by the Cochrane Review really means a lack of effect in some trials which could be due, as explained here, to the fact that anthelmintic treatment alone is not sufficient to achieve improved nutritional outcomes. The review received considerable criticism concerning the methods of analysis and conclusions (Bhargava 2000; Bundy and Peto 2000; Cooper 2000; Michael 2000; Savioli et al. 2000).
This review was withdrawn in 2007 and replaced by a new analysis that examined effects on growth and school performance (Taylor‐Robinson et al. 2007). The review drew similar conclusions: deworming may lead to improved weight gain in some circumstances but not others, and no effect on cognition or school performance has been demonstrated (Taylor‐Robinson et al. 2007).
6.4 Characteristics of an ideal study
Any studies that attempt to estimate the effect of anthelmintic treatment plus any supplement should ideally have a factorial design. This is illustrated in Fig. 25 in which four groups of subjects are given either deworming or not with a nutritional intervention or not, although it could be applied to any treatment. The factorial design is powerful, but requires randomization of subjects to study groups and an untreated control group to allow the effect of secular change to be estimated and subtracted from the effect of treatment.
However, it is important to appreciate that it may be possible to bring about an improvement in a nutritional variable by a nutritional intervention alone, so that the effect of deworming will be masked. An analogy of a leaking bucket of water may be helpful to explain this concept. The level of water in a bucket, which is the measured outcome, can be sustained or even raised if the water flowing into the bucket is greater than the loss through any leaks. Simply plugging the leaks will not raise the water level, it is necessary to add more water to achieve this. Adding water alone could overcome or mask the effect of the leaks and lead to a rise in the water level. Once the bucket is full, the excess simply overflows but the leaks continue. This creates a paradox: once you start to fill the bucket the change in the level of water no longer accurately reflects the leakage that is occurring.
This analogy may apply better to anaemia than to body weight, as the concentration of haemoglobin does not rise unchecked, although some excess nutrients such as iron and vitamin A can be stored. When worms cause a loss of blood and iron and the haemoglobin concentration falls, it will do so when all stores of iron needed to make haemoglobin have been used up (Crompton & Whitehead 1993). Treating hookworms will not lead to an increase in the haemoglobin concentration if the diet does not contain enough nutrients to supply normal turnover, which is estimated to be about 0.8% of all red blood corpuscles daily, and to provide for increased erythropoiesis to replace corpuscles lost caused by worms. But the cumulative loss of iron and other nutrients could be rectified by giving supplementary food alone, provided that all other requirements are met. The rate of recovery from anaemia is likely to be very slow without nutrient supplements, and studies of haemoglobin concentration after anthelmintic treatment may need to last for a year or more to show the full effect of treatment alone. The same could be said for measuring the effect of anthelmintic treatment on growth.
It would be helpful for any future meta‐analysis of the effects of anthelmintic treatments if any new research studies of the impact of treatment on outcomes such as growth or haemoglobin concentration had certain key features.
-
1
The study should be performed where worms and undernourished children are common. A prevalence of infections with intestinal nematode worms of more than 70% is suggested, and where >20% of children are underweight or >40% are anaemic.
-
2
A randomized, controlled design should be used. If individuals are randomized to treatment and control groups then a placebo should be used; if clusters are randomized, then a placebo is ideal, but not essential. To overcome the problem of not giving a treatment to subjects in a cluster trial without a placebo, both groups could be given some other treatment, such as vitamin A.
-
3
A sample size calculation should be done. As a rule of thumb there should be a minimum of 250 subjects in each group in a randomized trial. In a cluster trial there should be more than 25 clusters per group and a design effect of 2 should be applied.
-
4
Anthelmintic treatment should be given at least every 6 months. Ideally initial egg counts should be reduced by >70% by the first treatment. This could mean giving repeated treatment initially to achieve a high efficacy, unless an effectiveness trial is being performed.
-
5
If more than one anthelmintic is given, such as praziquantel with albendazole, or albendazole with micronutrient supplements, a 2 × 2 factorial design should be considered.
-
6
The key outcomes to be measured are weight and height. It may be helpful to measure triceps skinfold thickness and mid‐upper arm circumference to distinguish between increased adiposity and growth in tissues. If infections with hookworms or T. trichiura are common, then the haemoglobin concentration should be measured as well with, ideally, a measure of irons status such as ferritin or transferrin receptor (WHO/CDC 2005).
-
7
If growth is to be measured, the study should last a minimum of 1 year, and 2 years if possible. Specific criteria that state when a study should be stopped may need to be set.
-
8
If the haemoglobin concentration is to be measured the study should last a minimum of 12 weeks, and should be continued to 52 weeks if possible if no micronutrient supplements are given after treatment.
-
9
Drug efficacy should be assessed 21 days after each round of treatment as a matter of good practice, to assess the development of any anthelmintic resistance.
-
10
The gains in any parameter related to growth should be standardized as mean gain per year. The mean and standard deviation of all outcome measures and any derived indices should be given in any report or paper for each group, with the sample sizes. If subjects are studied in clusters, then this should be taken into account during the analysis.
6.5 Implications for programmes
Deworming is an easy and inexpensive thing to do, and the drugs are very safe. Treatment can be popular with parents (Brooker et al. 2001). Most anthelmintics can be given as a single dose so no adjustment for body weight is necessary, and a single 400 mg tablet of albendazole or 500 mg of mebendazole can be given to all children older than 1 year (WHO 2002b), although a syrup should be given to very young children. The United Nations Children Fund (UNICEF) in Ethiopia have issued warnings that young children should not be forced to take tablets because of the risk of choking. Anthelmintic drugs should not be given in the first trimester of pregnancy (WHO 1994b). Anthelmintic drugs are usually available over the counter and do not require a prescription, except in developed countries.
Over the last few years the costs of albendazole and mebendazole have been reduced to less than 5 US cents a tablet when bought in large quantities and, according to the WHO, these drugs can be given safely to all children once a year in areas where the prevalence of infection is more then 20% and twice a year where the prevalence is more than 50% (WHO 2006). The new recommendations are shown in Table 14. To promote mass deworming the World Health Assembly in 2001 passed resolution WHA54.19 asking countries to administer anthelmintic treatments annually to at least 75% of all school‐age children at risk of morbidity by 2010 (WHO 2002a).
Table 14.
The prevalence of any species of intestinal nematode that warrants mass treatment criteria for applying treatments and the target groups
| Risk category | Prevalence | Target groups and treatments | |
|---|---|---|---|
| High | ≥50% | Treat all school‐age children twice a year | Treat all: |
| • pre‐school children | |||
| • women of child‐bearing age except in first trimester | |||
| • adults at risk | |||
| Low | ≥20% to <50% | Treat all school‐age children once a year | Treat all: |
| • pre‐school children | |||
| • women of child‐bearing age except in first trimester | |||
| • adults at risk | |||
There are three justifications for giving mass treatment at least once a year in areas where the prevalence of infection with all species of intestinal nematode worms is greater than 50%.
First, because the probability of disease increases exponentially above a prevalence of 50% (see Fig. 3).
Second, the cost of diagnosis is typically many times the cost of treatment. When the prevalence is greater than 50% diagnosis becomes a matter of identifying uninfected people who do not need treatment, and so the cost per case of finding them increases with the prevalence.
Third, most anthelmintic drugs, and the benzimidazoles in particular, are very safe, and there is no known harm in treating people who are not infected. Benzimidazoles have been taken daily for long periods to treat hydatid disease (El‐On 2003; Horton 2003a) and when used to treat intestinal nematodes the overall frequency of side effects is about 1% (Horton 2000). Although one of the potential studies identified for the meta‐analysis reported evidence of an adverse effect on the growth of children given 400 mg of albendazole given for 3 days, there was no untreated control group (so the study was excluded from the present analysis) and the children treated with albendazole were compared with children treated with another anthelmintic, pyrantel embonate (Forrester et al. 1998).
The use of a 20% threshold at which to apply annual mass treatment in school‐age children has cost–benefit implications. When the prevalence of infection is less than 50% the majority of children are uninfected and perhaps only a few per cent of children will actually benefit from treatment. Although the cost per child treated may be small in terms of the cost of each tablet, as the prevalence drops the costs per infected child treated will increase. This is shown in Fig. 26 for a drug costing 3 US cents per treatment. When the prevalence is 50% the cost per infected child treated is twice the cost per child treated, or 6 US cents per child; when the prevalence is only 20% the cost per infected child treated is five times the cost per child treated or 15 US cents per child; and if only 5% of all children actually benefit from treatment the cost per beneficiary is 20 times the cost of the treatment or 60 US cents per child.
Figure 26.
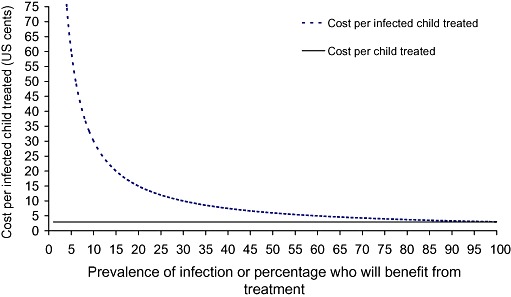
The costs of anthelmintic treatment per child for a drug costing 3 US cents per child and the costs per infected child treated.
Although these unit costs may seem small in absolute terms, the total cost to a government may be large. For example, if the Ethiopian government was considering treating the 11.5 million children enrolled in primary schools in 2005 with a drug costing 3 US cents per treatment, and if the prevalence of infection according to a recent national survey is 30% (SC/US 2007), then of the $345 000 it will cost to buy drugs, 70% or $241 500 will be spent on treating uninfected children.
These costs do not take into account the additional financial or opportunity costs of delivering and administering tablets.
The main issue for deworming programmes is how to deliver treatments at low cost to the three main groups who would benefit most from treatment: pre‐school children, school‐age children and women of reproductive age.
As there are many current programmes delivering treatments such as vaccinations and vitamin A to children less than 5 years, the additional cost of giving a tablet of albendazole or mebendazole is minimal. In recognition of this, the WHO and UNICEF now recommend deworming children as a part of all routine health programmes such as vitamin A supplementation (WHO/UNICEF 2004), and anthelmintic treatment is also recommend as a part of the Integrated Management of Childhood Illnesses after any acute illness has ceased.
A commonly unrecognized concern arises because of the lack of overlap between health programmes for pre‐school children, which typically stop at 5 years of age, and programmes for school children, which begin when children enrol in school. As the official age of enrolment in basic education in many countries is 6 or even 7 years of age, even if all children actually enrol in school at the correct age, there is a gap of 1–2 years when they may fall between programmes and miss out on treatment. As late‐enrolment in school is very common in sub‐Saharan Africa (Partnership for Child Development 1998b) and late enrolment is strongly associated with stunted growth and anaemia (Partnership for Child Development 1999b; Hall et al. 2001), the gap between programmes may be as much as 4 or 5 years, and such children may be in greater need of treatment than those who are actually in school.
In countries where enrolment rates are high, school health programmes offer an effective mechanism to reach a large proportion of school‐age children (Hall et al. 1996), an age group that harbours a large proportion of all worms in a community. The cost of delivering anthelmintics to school children has been shown to be a few US cents per child (Partnership for Child Development 1998a, 1999a). In addition to the potential nutritional benefits of treatment, periodically giving anthelmintics to school children alone has other benefits, as it can serve to bring down transmission to other, untreated groups (Asaolu et al. 1991; Butterworth et al. 1991; Bundy et al. 1992b). Such externalities, as they are called by economists, are not captured in a meta‐analysis such as this, which examines only one outcome at a time.
It is also possible that, in addition to affecting growth and micronutrient status, intestinal nematodes may affect children's cognitive function and educability, although the evidence for this is mixed and often comes from cross‐sectional associations, which are easily confounded (1992a, 1992b; Watkins & Pollitt 1997; Sakti et al. 1999; Dickson et al. 2000b; Jukes et al. 2002). The Cochrane Review of 2007 concluded that there was insufficient evidence that treating worms improves cognitive performance, which included educational outcomes including attendance and tests of educational achievements (Taylor‐Robinson et al. 2007). The controlled trial of 6‐monthly deworming for 2 years in Vietnam found no statistically significant difference between treatment and controls in terms of tests of mathematics and Vietnamese (Partnership for Child Development 2001).
The last group that would benefit considerably from periodic anthelmintic treatment are women of reproductive age, although treatment should only be given before pregnancy or after the first trimester. Nevertheless accidental treatment does not seem to be dangerous: a study of some 400 Sri Lankan women who had taken mebendazole during their first trimester of pregnancy did not find a greater rate of congenital defects than in pregnant women who had not taken the drug (de Silva et al. 1999). The sample size was too small to detect a greater risk of rare congenital defects, so the recommendation of the study was still to avoid treatment with anthelmintics (de Silva et al. 1999).
The main effect of anthelmintic treatment on pregnant women is likely to be on haemoglobin concentration as a result of treating infections with hookworm or Schistosoma spp. A randomized controlled trial in Sierra Leone of giving albendazole to pregnant women after their first trimester prevented a fall in haemoglobin concentration of 6.6 g L−1 (Torlesse & Hodges 2000, 2001). When albendazole was given with iron, both treatments prevented a fall in haemoglobin concentration of 13.7 g L−1 (Torlesse & Hodges 2000, 2001).
As a general rule for all programmes in which anthelmintic treatment for worms is given, drugs should not be administered to individuals who are sick for any reason. This is not because the drug may be ineffective, although it may be if there is diarrhoea. The reason for not giving treatment is because of the possibility that the subject may die of a pre‐existing illness, and it could be concluded that the anthelmintic had caused the death. In any programme in which very large numbers of children are being treated on the same day, such an event becomes increasingly statistically probable, so every effort should be made to ensure that only otherwise healthy children are treated. A possible example of this was the >30 deaths reported after the administration of vitamin A to some 3 million young children in the state of Assam in India in 2001 (West & Sommer 2002), although there was some concern that children may have been overdosed (Kapil 2002, 2004). Reports of deaths of school children during mass deworming have not been documented.
The other concern during mass treatment campaigns is for adolescent girls who may be pregnant and either not know it, or be afraid to report it. Teratogenic effects of drugs are most likely to occur during the first few weeks of pregnancy, a period when a woman may not yet be aware that she is pregnant. It cannot be assumed that school‐age girls are not sexually active. A study of 9000 school children in grades 4 to 6 in Tanzania found that 20% of girls reported having had sex, but only 39% of 114 girls with biological markers of sexual activity such as an infection acknowledged having had sex, indicating that such activity was greatly under‐reported (Todd et al. 2004). In an analysis of official education statistics, also in Tanzania, pregnancy was reported to be a cause of school dropout for six or seven girls per 1000 in grades 6 and 7 respectively (Partnership for Child Development 1998b).
These data should provide a warning to programmes that give mass treatment with anthelmintic drugs to school‐age children as well for those considering adding mega‐dose supplements of vitamin A to treat vitamin A‐deficient or anaemic adolescent girls. It cannot be assumed that adolescent girls are not pregnant.
6.6 Conclusions
Treating intestinal nematode worms can lead to significantly better weight gain and growth when treated children are compared with untreated controls.
The magnitude of the effect of treatment is specific to local epidemiological circumstances, and not all infected children will benefit to the same degree, if at all.
If no significant effect of anthelmintic treatment is detected in a study, it cannot be assumed that worms did not contribute to undernutrition; it is likely that removing worms is insufficient to lead to improved growth without extra food and nutrients.
The maximum size of any effect of treating worms cannot be estimated; studies can only indicate what difference is achievable. To show a significant effect it is necessary to have an untreated control group and a sufficient period of follow‐up. This is likely to become less tenable given the measured benefits of treating worms that have been shown. Nevertheless it would be useful to have more studies of the effectiveness of anthelmintics delivered through health programmes, especially those that focus on pre‐school and school‐age children, and perhaps given with multiple micronutrients to make sure that no vitamin or mineral is a factor limiting catch‐up growth.
Acknowledgements
This review was funded by the World Bank through the International Centre for Diarrhoeal Disease Research, Bangladesh. The principal author would like to acknowledge here his gratitude to David Crompton, Lani Stephenson and Michael Latham who, in 1978 in Kenya, introduced him to the study of human nutrition and parasitic worms.
Conflicts of interest
N. de Silva is a member of the Mebendazole Advisory Committee, an independent body of experts that advises Johnson & Johnson and the Mebendazole Donation Initiative. The remaining authors declare no conflicts of interest.
Appendix: Summary of papers identified for the review
Abbreviations used in the tables: CI, confidence intervals; DEC, diethyl carbamazine; DGLV, dark‐green leafy vegetables; epg, eggs per gram faeces; HAZ, z‐score of height‐for‐age; Ht, height; MUAC, mid‐upper arm circumference; NS, not statistically significant; SES, socio‐economic status; TDS, Trichuris dysentery syndrome; WAZ, z‐score of weight‐for‐age; WHZ, z‐score of weight‐for‐height; Wt, weight; y, years.
Notes
‘Included in Cochrane review of 2000’ means that the paper was used in the Cochrane Collaboration systematic review:
Dickson R, Awasthi S, Demellweek C, Williamson P (2000). Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance (Review). Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No. CD000371. doi:10.1002/14651858.CD000371
Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. Journal of Nutrition 124, 1199–1206.
| Design | Randomized controlled trial. |
| Infected children only. Paired according to hookworm egg count then randomized within pairs to treatment or placebo | |
| Follow‐up | 9 weeks |
| Location | Kwale, Kenya |
| Age range | 5–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 32% treatment; 26% controls |
| Trichuris trichiura: 79% treatment; 89% controls | |
| Hookworm: 93% treatment; 93% controls | |
| Treatments | Albendazole 400 mg day−1 over 3 days vs. Placebo |
| Sample size | Albendazole 28 |
| Placebo 27 | |
| Outcomes | Weight change Z‐score of weight‐for‐age change |
| Height change Z‐score of height‐for‐age change | |
| MUAC change Z‐score of weight‐for‐height change | |
| Triceps change | |
| Haemoglobin change | |
| Findings | |
| – Weight change, kg | |
| Albendazole 1.0 SE 0.06 | |
| Placebo 0.3 SE 0.10 P = 0.0002 | |
| – Height change, cm | |
| Albendazole 0.9 SE 0.10 | |
| Placebo 0.8 SE 0.11 P = 0.24 | |
| – MUAC change, cm | |
| Albendazole 0.6 SE 0.07 | |
| Placebo 0.3 SE 0.05 P = 0.0002 | |
| – Triceps change, mm | |
| – Albendazole 1.0 SE 0.13 | |
| – Placebo 0.2 SE 0.09 P = 0.0002 | |
| – Haemoglobin change, g L−1 | |
| Albendazole −1.6 SE 1.9 | |
| Placebo −2.6 SE 1.6 NS within groups | |
| – Z‐score of weight‐for‐age change | |
| Albendazole 0.3 SE 0.024 | |
| Placebo 0.08 SE 0.034 P = 0.0002 | |
| – Z‐score of height‐for‐age change | |
| Albendazole 0.016 SE 0.017 | |
| Placebo 0.016 SE 0.017 P = 0.42 | |
| – Z‐score of weight‐for‐height change | |
| Albendazole 0.33 SE 0.036 | |
| Placebo −0.03 SE 0.06 P = 0.0002 | |
| Notes | |
| – Subjects severely anaemic (haemoglobin ≤75 g L−1) excluded | |
| – At baseline mean z‐score of height‐for‐age was −1.35 placebos and −1.48 albendazole; mean z‐score of weight‐for‐height was −0.27 placebos and −0.66 albendazole (Table 2) | |
| – Also looks at activity and appetite after treatment; there was more free play in the treated children and increased self‐reported appetite (page 1202) | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Effect on weight gain of routinely giving albendazole to preschool children during child health days in Uganda: cluster randomized controlled trial. British Medical Journal 333, 122–124.
| Design | Cluster randomized controlled trial of effectiveness; not blinded |
| Follow‐up | Variable; minimum 6 months, max. >2 y |
| Location | Eastern Uganda; rural |
| Age range | 1–7 y |
| Infection prevalence at baseline | Survey of young school children in the same region before study found a prevalence of A. lumbricoides 17.5%, T. trichiura 7.3%, hookworms 44.5% and 56% any infection (Kabatereine et al. 2001) |
| Treatments | Albendazole 400 mg at biannual Child Health Dayvs. No albendazole |
| Sample size | Albendazole 14 940 |
| No albendazole 13 055 | |
| Outcomes | Weight gain |
| Findings | |
| – Mean weight gain in treated children 2.413 SD 2.536 kg | |
| – Mean weight gain in untreated children 2.259 SD 2.474 kg | |
| – Significant difference of 154 g; P < 0.01 | |
| – Equivalent to extra 166 g y−1 or nearly 10% extra of initial weight | |
| – Children treated twice a year gained more weight than those treated less often (Table 3, model 3; see also model 6) | |
| Notes | |
| – Programme ran for 31.75 months; mean time in programme was 16.6 SD 7.6 months and mean visit to Child Health Days was 2.7 visits SD 0.9 | |
| – Some control children were dewormed elsewhere (24% at baseline and 35% at end – Table 4) so effect may be underestimated | |
| – ∼25% of children were underweight at baseline | |
| – Predominant helminth in area is hookworm, estimated from survey of young school children, see Kabatereine et al. (2001) East African Medical Journal 78: 283–286. | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Effectiveness and cost‐effectiveness of albendazole in improving nutritional status of pre‐school children in urban slums. Indian Pediatrics 37, 19–29.
| Design | Randomized controlled trial. Single blind. 32/150 slums randomly selected, then all eligible children randomized to treatment/placebo. No selection on basis of worm infections |
| Follow‐up | 2 y |
| Location | Lucknow, India; urban slums |
| Age range | 1.5–3.5 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 12.5% treatment; 10.6% controls |
| Trichuris trichiura: not reported | |
| Hookworm: 2.6% treatment; 4.9% controls | |
| Treatments | Albendazole 600 mg at usual 6‐monthly health visits vs. Placebo |
| Sample size | Albendazole 601 |
| Placebo 444 At last follow‐up | |
| Outcomes | Weight gain % underweight (z‐score) |
| Height gain % stunted (z‐score) | |
| Haemoglobin % wasted (z‐score) | |
| Findings | |
| – Weight gain 2.68 SD 1.2 kg in placebo | |
| Weight gain 2.63 SD 1.34 kg in albendazole | |
| – Height gain 10.35 SD 5.1 cm in placebo | |
| Height gain 9.94 SD 4.9 cm in albendazole | |
| – Differences in gains NS | |
| – Haemoglobin gain 9.67 (given as g dL−1 in paper but must be g L−1) in both groups; method not stated and no SD given | |
| – % underweight and % wasted fell significantly in both groups; % stunted rose significantly in placebo but did not change significantly in albendazole group (Table II); difference in the % change between groups was 9.38% (significant) | |
| Notes | |
| – Haemoglobin estimated by visual colour estimation using a haemoglobinometer. Method not validated against haemoglobin level (page 3) | |
| – At baseline about 4% had hookworm and 11% Ascaris; intensity of infections not estimated (Table I); placebo group had significantly more hookworm at baseline (4.9% vs. 2.6%, P = 0.05) | |
| – At baseline about 58% were stunted, 18% wasted and 90% anaemic (Tables I, II) | |
| – Some placebo children given treatment (∼10%) (page 5) | |
| – In Discussion says sample size too small for detecting height gains (page 9) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: low prevalence of worms | |
Six‐monthly de‐worming in infants to study effects on growth. Indian Journal of Pediatrics 68, 823–827.
| Design | Randomized controlled trial, cluster randomized.Vitamin A vs. vitamin A + albendazole. 124/200 slums randomly allocated to two groups. All children within a cluster included. Not blinded |
| Follow‐up | 18 months |
| Location | Lucknow, India; urban slums |
| Age range | 6–12 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 8% albendazole; 9% vitamin A |
| Trichuris trichiura: Not reported | |
| Hookworm: Not reported | |
| Treatments | Albendazole 400 mg + vitamin A (100 000 units) at usual 6‐monthly health visits vs. Vitamin A (100 000 units) |
| Sample size | Albendazole + vitamin A 832 |
| Vitamin A 840 (At 18 months) | |
| In children with history of passing worms: | |
| Albendazole + vitamin A 301 | |
| Vitamin A 143 | |
| Outcomes | Weight gain |
| Height gain | |
| Findings | |
| – Weight gain 3.22 kg (0.26 SE) in albendazole + vitamin A | |
| Weight gain 3.05 kg (0.19 SE) in vitamin A P = 0.01 | |
| – Height gain 16.5 cm (0.82 SE) in albendazole + vitamin A | |
| Height gain 16.1 cm (0.83 SE) in vitamin A P = 0.2 | |
| – In children with history of passing worms (Table 3): | |
| Weight gain 3.28 kg (0.17 SE) in albendazole + vitamin A | |
| Weight gain 2.90 kg (0.17 SE) in vitamin A P = 0.007 | |
| Notes | |
| – At baseline about 9% had Ascaris; no measurements of intensity made | |
| – At baseline mean z‐score of weight‐for‐age was −1.98, z‐score of height‐for‐age was −2.43 and z‐score of weight‐for‐height was −0.08 and prevalence of underweight ∼47%, stunting ∼62% and wasting ∼17% | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: low prevalence of worms | |
Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian schoolchildren. American Journal of Tropical Medicine and Hygiene 60, 479–486.
| Design | Randomized controlled trial. Double blind. Five schools; all children randomly assigned into four groups |
| Follow‐up | 4 months |
| Location | Leogane, Haiti; coastal town |
| Age range | 5–11 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 28.3% albendazole, 24.1% ivermectin, 33.5% both treatments, 31% control |
| Trichuris trichiura: 42.5% albendazole, 40.7% ivermectin, 42.7% both treatments, 43% control | |
| Hookworm: 5.5% albendazole, 6.5% ivermectin, 7.8% both treatments, 8% control | |
| Treatments | Albendazole 400 mg |
| vs. Ivermectin 200–400 µg kg−1 | |
| vs. Albendazole + ivermectin | |
| vs. Placebo (vitamin C) | |
| Sample size | Children with hookworm: With Trichuris only: |
| Albendazole 12 42 | |
| Ivermectin 14 46 | |
| Albendazole + ivermectin 17 34 | |
| Placebo 16 36 | |
| Outcomes | Weight change Z‐score of weight‐for‐age change |
| Height change Z‐score of height‐for‐age change | |
| Z‐score of weight‐for‐height change | |
| Findings | |
| – States there was no nutritional benefit for the whole study population (n = 853; Fig. 1), nor any benefit in weight gain, height gain or other anthropometric indices when only the 54.7% with infections were analysed | |
| – No benefit in children with Ascaris only | |
| Hookworm Trichuris only | |
| (not exclusively) | |
| Weight change, kg | |
| Albendazole 0.89 0.95 | |
| Ivermectin 0.87 0.73 | |
| Both 0.76 1.27 P = 0.01 | |
| Placebo 0.67 0.71 | |
| Height change, cm | |
| Albendazole 1.69 1.83 | |
| Ivermectin 1.86 1.80 | |
| Both 1.98 1.79 P = 0.01 | |
| Placebo 1.36 1.90 | |
| Z‐score of weight‐for‐age change | |
| Albendazole 0.035 0.035 | |
| Ivermectin 0.010 −0.002 | |
| Both 0.006 0.120 P = 0.04 | |
| Placebo 0.008 −0.009 | |
| Hookworm Trichuris only | |
| (not exclusively) | |
| Z‐score of height‐for‐age change | |
| Albendazole −0.020 0 | |
| Ivermectin 0.020 −0.003 | |
| Both 0.028 −0.007 P = 0.04 | |
| Placebo −0.080 0.015 | |
| Z‐score of weight‐for‐height change | |
| Albendazole 0.063 0.071 | |
| Ivermectin 0.018 0.001 | |
| Both −0.007 0.194 P = 0.01 | |
| Placebo 0.071 −0.029 | |
| Note: P‐values in comparison with placebo group | |
| Notes | |
| – >96% of infections were classified by the authors as light (Ascaris <7000 eggs g−1; Trichuris <1000 eggs g−1; hookworm <2000 eggs g−1) | |
| – Both treatments effectively decreased the prevalence and intensity of Ascaris (Table 1). Albendazole was 100% effective against hookworm, but only reduced the prevalence, not the intensity, of Trichuris | |
| – At baseline, mean z‐score of height‐for‐age −0.43, z‐score of weight‐for‐age −0.53, z‐score of weight‐for‐height −0.37; 5% had mild anaemia | |
| – Authors suggest adequate baseline nutritional status and light intensity infections may explain the lack of effect | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no standard deviations given | |
The impact of population level deworming on the haemoglobin levels of schoolchildren in Tanga, Tanzania. Tropical Medicine and International Health 4, 744–750.
| Design | Randomized controlled trial. |
| Randomization on basis of prevalence and intensity of infections. Children with S. haematobium and at least one geohelminth included. Single blind | |
| Follow‐up | 4 months |
| Location | Muheza District, Tanzania; 3 rural villages |
| Age range | 7–12 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 47% treatment; 51% controls |
| Trichuris trichiura: 69% treatment; 67% control | |
| Hookworm: 94% treatment; 92% control | |
| Treatments | Albendazole 400 mg + praziquantel 40 mg kg−1 single dose |
| vs. Placebo | |
| Sample size | Albendazole + praziquantel 127 |
| Placebo 123 | |
| Outcomes | Haemoglobin % change |
| Findings | |
| – % change in mean haemoglobin (g dL−1) −0.11 SD 0.07 treatment group | |
| – % change in mean haemoglobin (g dL−1) −0.35 SD 0.07 placebo group | |
| – P = 0.02 | |
| – But no significant difference in % change of anaemia prevalence (Table 5) | |
| – The greatest effect of treatment on haemoglobin was seen in anaemic children with heavy hookworm loads. No effect on haemoglobin was seen in non‐anaemic children (Tables 7,8) | |
| Notes | |
| – All subjected were infected with S. haematobium | |
| – ∼48% were anaemic | |
| – After treatment infection prevalences fell but remained at 54% hookworm, 52% Trichuris and 12% Ascaris | |
| – Hookworm mean egg counts fell by 81% to 430 eggs g−1 | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: praziquantel given with albendazole | |
Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food and Nutrition Bulletin 24, 332–342.
| Design | Children randomized to treatment or control. Controls used in a longitudinal analysis of infection and haemoglobin (page 335). Treatment group divided into high, medium and low infections. Low considered ‘uninfected’ and used for comparisons, high were treated at baseline and medium were not used. At 3 months low and high infected children were treated if needed. Treatment was tailored to infection where given (see Fig. 1) |
| Follow‐up | 3 and 15 months |
| Location | Bagamoyo and Kibaha districts (coastal); 10 schools |
| Age range | 9–15 y |
| Infection prevalence at baseline | Ascaris lumbricoides: Not reported |
| Trichuris trichiura: Not reported | |
| Hookworm: Treatment 100% divided into heavy (>400 eggs g−1) or ‘uninfected’ <50 eggs g−1; controls: not screened | |
| Schistosoma haematobium: Treatment 100% divided into heavy >50 eggs per 10 mL of urine or ‘uninfected’ <5 eggs per 10 mL | |
| Controls: Unknown as subjects not screened | |
| Treatments | Albendazole 400 mg for 3 days + praziquantel 40 mg kg−1 as required (see Fig. 1) |
| vs. B group vitamins | |
| Sample size | Controls 729 (453 in Table 3) |
| Uninfected (low) 116 | |
| Infected (high) 270 | |
| Note: n falls at each survey round (Fig. 1), but presumably means in Table 2 are for all children measured at each round, not just those children for whom three measurements are available | |
| Outcomes | Haemoglobin |
| Findings | |
| – Says albendazole had a significant impact on haemoglobin (anova) (p. 337) | |
| – Infected children given albendazole: baseline haemoglobin 111.2 SD 16.4 g L−1; 3 month 113.6 SD 12.6 g L−1; 15 month 120.5 SD 12.6 g L−1 (P < 0.001 baseline vs. 15 months) | |
| – Uninfected children given albendazole at 3 months: baseline haemoglobin 119.0 SD 11.7 g L−1; 3 month 116.4 SD 10.2 g L−1; 15 month 121.7 SD 12.7 g L−1 (P = NS? baseline vs. 15 months) | |
| – Interaction between praziquantel and albendazole not significant | |
| Notes | |
| – Treatment effects on haemoglobin were tested separately for treatment and ‘control’ groups (Tables 2,3). But the ‘controls’ are grouped by their infection status at 15 m and the infected by infection status at baseline | |
| – In Tables 2 and 3 we do not know if uninfected children are comparable to infected children because they were all put together in Table 1. They might have a higher SES etc. | |
| – Last two paragraphs of second section of results make their case for using the control group as genuine controls – the problem is that the infection status of the controls wasn't measured until 15 months. They state hookworm treatment improves haemoglobin in infected children, but there are no statistics comparing treatment and control (page 339) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: praziquantel given with albendazole; controls provided by children who were either lightly infected or uninfected | |
Treatment effects in Trichuris dysentery syndrome. Acta Paediatrica 83, 1182–1187.
Further follow‐up in Acta Paediatrica 87, 1247–1249.
| Design | Case–control study. |
| Hospitalized children with TDS matched with controls who lived near case children and had no Trichuris | |
| Follow‐up | 1 y |
| Location | Kingston, Jamaica |
| Age range | 27–84 months |
| Infection prevalence at baseline | Ascaris lumbricoides: Not reported |
| Trichuris trichiura: Treatment 100% with Trichuris dysentery syndrome; 0% in controls | |
| Hookworm: Not reported | |
| Treatments | Mebendazole 200 mg for 3 days every 3 months for 1 y |
| vs. No treatment | |
| Treated children also received iron (24 mg) for 1 month on discharge | |
| Sample size | Mebendazole 19 |
| Controls 18 | |
| At 4 y follow‐up: 18 mebendazole and 17 controls | |
| Outcomes | Haemoglobin Z‐score of height‐for‐age |
| MUAC Z‐score of weight‐for‐height | |
| Findings | |
| – MUAC, cm | |
| 14.2 SD 1.2 to 15.9 SD 1.0 TDS group | |
| 15.9 SD 1.1 to 16.4 SD 1.3 control group | |
| – Haemoglobin g L−1 | |
| 80.0 SD 24.0 to 107.0 SD 12.0 TDS group | |
| 114.0 SD 8.0 to 114.0 SD 10.0 control group | |
| – Z‐score of height‐for‐age | |
| −2.2 SD 1.1 to −1.2 SD 0.8 TDS group | |
| −0.2 SD 1.1 to −0.2 SD 1.1 control group | |
| – Z‐score of weight‐for‐height | |
| −1.4 SD 1.0 to −0.7 SD 0.7 TDS group | |
| −0.6 SD 0.9 to −0.6 SD 1.0 control group | |
| – All P = 0.0001 for ancova group × measurement session (Table 2) | |
| – At 4 y follow‐up mean values were: | |
| MUAC, cm 17.8 SD 1.3 TDS 18.5 SD 1.9 control | |
| Haemoglobin, g L−1 113 SD 8.0 TDS 110 SD 8 control | |
| Z‐score of height‐for‐age −0.3 SD 0.8 TDS 0.02 SD 1.1 control | |
| Notes | |
| – Results are also given for follow‐ups at 7.5 weeks and 6 months – Table 2 | |
| – See Table 2 for ancova statistics | |
| – Authors comment on impressive height catch‐up given children's poor home circumstances and no supplementary food | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: not a randomized trial; controls inadequate | |
Vitamin A supplementation but not deworming improves growth of malnourished preschool children in eastern Zaire. Journal of Nutrition 128, 1320–1327.
| Design | Randomized controlled trial of children discharged consecutively from hospital (after treatment for protein‐energy malnutrition?) |
| Follow‐up | 1 y |
| Location | South Kivu province, Zaire |
| Age range | 0–72 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 9.6% mebendazole, 14.2% vitamin A, 10.5% controls |
| Trichuris trichiura: 2.8%–4.8% | |
| Hookworm: Not found | |
| Treatments | Mebendazole 500 mg single dose every 3 months |
| vs. Vitamin A (60 mg) at discharge and 6 months | |
| vs. No treatments | |
| Sample size | Final sample size for anthropometric is 236 but does not say how many in each group; at baseline: |
| Mebendazole 123 | |
| Vitamin A 118 | |
| No treatments 117 | |
| Outcomes | Weight gain |
| Height gain | |
| MUAC gain | |
| Findings | |
| – Means given and what are assumed to be 95% CI | |
| – Weight gain, kg (adjusted) | |
| Mebendazole group 1.715 (1.474–1.956) P = 0.002 vs. control | |
| Vitamin A group 2.410 (2.167–2.653) NS vs. control | |
| Controls 2.266 (2.019–2.513) | |
| – Height gain, cm (adjusted) | |
| Mebendazole group 7.51 (6.74–8.28) P = 0.028 vs. control | |
| Vitamin A group 8.47 (7.69–9.27) NS vs. control | |
| Controls 8.75 (7.96–9.54) | |
| – MUAC gain, cm (adjusted) | |
| Mebendazole group 0.60 (0.34–0.85) P = 0.012 vs. control | |
| Vitamin A group 1.39 (1.13–1.64) NS vs. control | |
| Controls 1.07 (0.81–1.33) | |
| – Mebendazole group who had serum retinol >0.35 µmol at baseline gained significantly less weight and MUAC than controls at 12 months (Tables 2,4) | |
| Notes | |
| – Results are adjusted for age, sex, serum albumin and serum retinol. | |
| – Results are also given for 0–6‐month interval and 6–12‐month interval for all the outcomes; weight results are also broken down by gender and initial serum retinol, height results also by gender and MUAC results also by initial serum retinol (Tables 2–4) | |
| – At 12 months % Ascaris infected was not different between the groups – it rose to ∼55% in all (Table 5). Median Ascaris egg count was also not different between groups by end, but the proportion of children with a light infection (<5000 eggs g−1) was significantly greater at 12 months: 85% vs. ∼60% | |
| ? effectiveness of treatment | |
| – At baseline ∼55% had z‐score of weight‐for‐age <−2, ∼65% had z‐score of height‐for‐age <−2, and ∼6% had z‐score of weight‐for‐height <−2; only ∼25% had normal serum retinol values | |
| – Only control and mebendazole groups used In Cochrane (2000) | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: No: low prevalence of worms | |
Impact of iron supplementation and deworming on growth performance in preschool Beninese children. European Journal of Clinical Nutrition 55, 223–228.
| Design | Randomized controlled trial. Double blind.Iron and/or albendazole. All children included |
| Follow‐up | 3 and 10 months |
| Location | Department de l’Ouémé; semi‐rural |
| Age range | 3–5 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 31% in albendazole‐treated groups; 47% in non‐albendazole‐treated groups |
| Trichuris trichiura: 38% in albendazole‐treated groups; 59% in non‐albendazole‐treated groups | |
| Hookworm: 11% in albendazole‐treated groups; 14% in non‐albendazole‐treated groups | |
| Treatments | Iron and albendazole |
| vs. Iron and placebo | |
| vs. Albendazole and placebo | |
| vs. Placebos | |
| Iron daily for first 3 months; 60 mg elemental iron | |
| Albendazole at baseline and at 1 month; 200 mg for 3 days | |
| Sample size | Anthropometry Anthropometry Haemoglobin |
| 3 m 10 m | |
| Iron and albendazole 34 31 34 | |
| Iron and placebo 36 33 34 | |
| Albendazole and placebo 38 37 38 | |
| Placebos 32 28 32 | |
| Outcomes | Weight change Wasting (z‐score) |
| Height change Stunting (z‐score) | |
| MUAC change | |
| Triceps skinfold change | |
| Haemoglobin change | |
| Findings | |
| Weight change, kg at 3 months at 10 months | |
| Iron and albendazole 0.4 SD 0.4 1.2 SD 0.6 | |
| Iron alone 0.3 SD 0.4 1.2 SD 0.5 | |
| Albendazole alone 0.4 SD 0.4 1.2 SD 1.0 | |
| Placebos 0.5 SD 0.6 1.2 SD 1.1 | |
| Height change, cm at 3 months at 10 months | |
| Iron and albendazole 1.9 SD 0.6 6.2 SD 1.0 | |
| Iron alone 1.9 SD 0.6 6.2 SD 1.5 | |
| Albendazole alone 2.3 SD 0.8 6.5 SD 2.6 | |
| Placebos 2.1 SD 0.9 6.0 SD 2.5 | |
| MUAC change, cm at 3 months at 10 months | |
| iron and albendazole −0.1 SD 0.6 0.1 SD 0.7 | |
| iron alone −0.2 SD 0.6 0.0 SD 0.8 | |
| Albendazole alone −0.2 SD 0.6 0.1 SD 0.8 | |
| Placebos 0.0 SD 0.8 0.1 SD 0.9 | |
| Triceps change, mm at 3 months at 10 months | |
| Iron and albendazole 0.4 SD 1.1 0.0 SD 1.5 | |
| Iron alone 0.0 SD 1.1 −0.2 SD 1.6 | |
| Albendazole alone 0.3 SD 1.6 −0.6 SD 1.3 | |
| Placebos 0.5 SD 1.2 0.2 SD 1.7 | |
| Haemoglobin (g L−1) at 3 months at 10 months | |
| Iron and albendazole 8 SD 3 13 SD 15 | |
| Iron alone 7 SD 14 11 SD 12 | |
| Albendazole alone 2 SD 16 8 SD 13 | |
| Placebos 4 SD 10 5 SD 12 | |
| – No significant differences for the anthropometry, including z‐scores | |
| – For haemoglobin, significant difference between treatment groups at 3 and 10 months (anova, P < 0.05) (page 225) | |
| Notes | |
| – Treatment achieved a 69% reduction in geometric mean hookworm eggs g−1, 74% for Ascaris and 49% for Trichuris | |
| – At baseline 76% were anaemic, 58% stunted, 2% wasted | |
| – Standard deviations were very large | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes; used data at 10 months, not 3 months after treatment | |
Effect of Ascaris lumbricoides infestation on growth of children. Indian Pediatrics 20, 721–731.
| Design | Randomized controlled trial. Two villages randomized to treatment and control |
| Follow‐up | 6 y |
| Location | Sri Lanka; rural |
| Age range | 2–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 82% treatment; 73% control |
| Trichuris trichiura: Not reported | |
| Hookworm: 47% | |
| Treatments | Piperazine citrate 10–30 mL every 2 months |
| vs. No treatment | |
| Only infected children or those who did not give a stool sample were treated | |
| Sample size | Wt & Ht 4 y MUAC 3 y MUAC 7 y |
| Piperazine 13 M; 15 F 19 M; 13 F 20 M; 16 F | |
| No treatment 16 M; 17 F 15 M; 13 F 14 M; 15 F | |
| Outcomes | Weight – in 4‐y‐olds |
| Height – in 4‐y‐olds | |
| MUAC – in 3‐ and 7‐y‐olds | |
| Findings | |
| – Weight in 4‐y‐olds, kg | |
| Boys | |
| Piperazine 13.07 (1.40) to 20.16 (1.21) | |
| No treatment 13.48 (1.56) to 21.46 (2.46) | |
| Girls | |
| Piperazine 12.14 (1.06) to 20.86 (3.13) | |
| No treatment 12.32 (1.22) to 18.65 (3.83) | |
| – Height in 4‐y‐olds, cm | |
| Boys | |
| Piperazine 94.6 (5.12) to 119.8 (4.95) | |
| No treatment 99.0 (4.87) to 122.8 (5.66) | |
| Girls | |
| Piperazine 94.6 (4.38) to 121.1 (7.88) | |
| No treatment 94.3 (4.08) to 116.2 (8.32) | |
| – MUAC 3‐y‐olds, cm | |
| Boys | |
| Piperazine 15.4 (0.9) to 16.1 (1.1) | |
| No treatment 15.3 (0.8) to 15.7 (1.2) | |
| Girls | |
| Piperazine 15.3 (1.5) to 16.9 (1.6) | |
| No treatment 15.3 (1.2) to 15.8 (1.8) | |
| – MUAC 7‐y‐olds, cm | |
| Boys | |
| Piperazine 15.7 (0.8) to 18.4 (1.7) | |
| No treatment 15.8 (0.8) to 18.3 (1.3) | |
| – Girls | |
| Piperazine 16.2 (1.0) to 19.0 (1.7) | |
| No treatment 15.7 (1.3) to 17.1 (1.7) | |
| – No P‐values given for height and weight measurements; states no tests on weight were significant and 5/96 on height were significant. | |
| – For MUAC, significant differences for girls after 3 y of study, vs. controls? (Table V) | |
| Notes | |
| – Treatment reduced Ascaris prevalence to 21% | |
| – Not used in Cochrane meta‐analysis because data segregated by sex and not all age groups shown | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: subjects were allocated to a treatment or control village, so sample size was only two; data were presented disaggregated by sex. Weak design. | |
Randomised trial of albendazole and pyrantel in symptomless trichuriasis in children. Lancet 352, 1103–1108.
| Design | Block randomized trial of 2 albendazole regimes and pyrantel. Only Trichuris‐infected children included, but none with TDS. No untreated group. Double blind bar 1 person |
| Follow‐up | 1 y |
| Location | Coatzacoalcos, Mexico; urban |
| Age range | 2–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 61% albendazole 1200 mg; 66% albendazole 400 mg; 61% pyrantel |
| Trichuris trichiura: 100% in all groups | |
| Hookworm: 7% albendazole 1200 mg; 7% albendazole 400 mg; 6% pyrantel | |
| Treatments | Albendazole 3 days at 400 mg = 1200 mg |
| vs. Albendazole 1 day at 400 mg + 2 days placebo | |
| vs. Pyrantel 1 day at 11 mg kg−1 + 2 days placebo | |
| Treated at baseline, 4 and 8 months | |
| Sample size | All Highest 25% infants Lowest 75% infants |
| Albendazole‐1200 174 37 137 | |
| Albendazole‐400 160 42 118 | |
| Pyrantel 174 48 126 | |
| Outcomes | Weight gain |
| Height gain | |
| MUAC gain | |
| Triceps gain | |
| Findings | |
| – All children: | |
| Pyrantel Albendazole Albendazole | |
| 400 mg 1200 mg | |
| Wt change, kg 2.92 SD 1.88 2.91 SD 1.69 2.74 SD 1.64 | |
| Ht change, cm 6.39 SD 1.33 6.53 SD 1.32 6.43 SD 1.37 | |
| MUAC change, cm 0.33 SD 0.63 0.32 SD 0.62 0.24 SD 0.64 | |
| Triceps change, mm 0.4 SD 1.28 0.25 SD 1.15 0.074 SD 1.23 | |
| – All NS except triceps where albendazole‐1200 had a significantly lower increase vs. pyrantel group. Difference −0.32 mm, P = 0.0096 | |
| – Highest 25% of infections: | |
| Pyrantel Albendazole Albendazole | |
| 400 mg 1200 mg | |
| Wt change, kg 2.63 SD 1.56 2.63 SD 1.43 2.96 SD 1.65 | |
| Ht change, cm 6.09 SD 1.48 6.50 SD 1.02 6.27 SD 1.32 | |
| AC change, cm 0.25 SD 0.64 0.31 SD 0.68 0.51 SD 0.66 | |
| Triceps change, mm 0.38 SD 1.20 0.29 SD 1.09 0.52 SD 1.45 | |
| – All NS except MUAC where albendazole‐1200 had a significantly greater increase vs. pyrantel group. Difference 0.26 cm, P = 0.044 | |
| – Lowest 75% of infections: | |
| Pyrantel Albendazole Albendazole | |
| 400 mg 1200 mg | |
| Wt change, kg 3.04 SD 1.97 3.00 SD 1.76 2.70 SD 1.62 | |
| Ht change, cm 6.50 SD 1.25 6.54 SD 1.41 6.49 SD 1.38 | |
| AC change, cm 0.36 SD 0.63 0.33 SD 0.60 0.18 SD 0.63 | |
| Triceps change, mm 0.40 SD 1.31 0.24 SD 1.16 −0.014 SD 1.15 | |
| – Ht changes NS but significantly less growth in albendazole‐1200 for weight (difference −0.33 kg, P = 0.036), MUAC (difference −0.18 cm, P = 0.0095) and triceps (difference −0.41 mm, P = 0.0031) | |
| – In regression analysis pre‐treatment level of infection had an effect in pyrantel and albendazole‐400 groups, but not albendazole‐1200 (page 1106) | |
| Notes | |
| – Hypothesis was that a higher dose = more worms removed = more growth in response to treatment. A placebo group was considered unethical given high prevalence of Ascaris | |
| – At baseline ∼16% had Giardia lamblia | |
| – Geometric mean egg count of Trichuris reduced by 99% in albendazole‐1200, by 87% in albendazole‐400 and by 67% in pyrantel (significantly different between groups) | |
| – Suggests that at low levels of Trichuris infection albendazole‐1200 group grew less, while at high but symptomless levels of infection, albendazole‐1200 resulted in better growth | |
| – Study generates controversy (see subsequent letters to Lancet) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no untreated control group | |
Tolerance and efficacy of combined diethyl carbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. American Journal of Tropical Medicine and Hygiene 73, 115–121.
| Design | Randomized controlled trial of either or both drugs vs. placebo. Double blind. All children included |
| Follow‐up | 6 months |
| Location | Leogane, Haiti; coastal town |
| Age range | 5–11 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 30.7% |
| Trichuris trichiura: 53.4% | |
| Hookworm: 9.7% | |
| Wuchereria bancrofti: 15.8% | |
| One or more parasites: 69.9% | |
| Treatments | Diethyl carbamazine (DEC) 6 mg kg−1 and/or albendazole 400 mg as single doses |
| vs. Vitamin C placebo | |
| Sample size | All Trichuris only |
| Albendazole 320 98 | |
| DEC 313 111 | |
| Albendazole + DEC 310 98 | |
| Placebo 306 93 | |
| Outcomes | Weight gain |
| Height gain | |
| Findings | |
| – No significant differences in anthropometric in children with hookworm or Ascaris, but in those with Trichuris only: | |
| – Height gain, cm | |
| Placebo 3.5 | |
| Albendazole 3.48 | |
| DEC 3.38 | |
| DEC‐Albendazole 3.26 Not significant | |
| – Weight gain, kg | |
| Placebo 1.16 | |
| Albendazole 1.44 Albendazole vs. placebo P = 0.038 | |
| DEC 1.39 | |
| DEC‐albendazole 1.47 DEC‐albendazole vs. placebo P = 0.046 | |
| – Weight gains over placebo were 0.28 kg albendazole and 0.31 kg DEC‐albendazole | |
| – Albendazole also improved z‐score of weight‐for‐age but not z‐score of height‐for‐age or z‐score of weight‐for‐height (z‐score data not shown) | |
| Notes | |
| – The focus of this paper is on the effectiveness of the two drugs at treating microfilaraemia and helminths (Tables 2,3) and the benefit of adding albendazole to filariasis control programmes | |
| – >90% of all infections were estimated to be light (<7000 eggs g−1 Ascaris, <1000 eggs g−1 Trichuris, <2000 eggs g−1 hookworm). | |
| – Albendazole and DEC‐albendazole both significantly decreased Ascaris prevalence and intensity by ≥93% vs. DEC and placebo; albendazole and DEC‐albendazole significantly reduced hookworm by ≥80%; and likewise Trichuris, but reduction only ∼32% (Table 3) | |
| – Authors suggest low helminth infection intensities responsible for lack of effects on anthropometric measurements | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no standard deviations given for Trichuris results. Anthropometry results for whole group not given in paper | |
Freij et al. (1979 ) (Trial I)
Ascariasis and malnutrition: a study in urban Ethiopian children. American Journal of Clinical Nutrition 21, 1545–1553.
| Design | Randomized controlled trial. |
| Ascaris‐infected boys only. Excluded if harbouring other infections. Boys paired by age and nutritional status then within pairs randomly allocated to treatment or placebo. Double blind? | |
| Follow‐up | 28 days |
| Location | Addis Ababa, Ethiopia |
| Age range | 1.5–5 y; boys only |
| Infection prevalence at baseline | Ascaris lumbricoides: 100% |
| Trichuris trichiura: None | |
| Hookworm: None | |
| Treatments | Piperazine citrate 3 g single dose |
| vs. Placebo | |
| Sample size | Piperazine 6 |
| Placebo 7 | |
| Outcomes | Weight (also weight % standard) |
| Triceps | |
| MUAC | |
| Findings | |
| – Weight change, kg | |
| Piperazine 0.27 SD 0.27 | |
| Placebo 0.27 SD 0.22 | |
| – MUAC change, cm | |
| Piperazine 0.07 SD 0.90 | |
| Placebo 0.44 SD 0.20 | |
| – Triceps change, mm | |
| Piperazine 2.2 SD 2.26 | |
| Placebo 2.31 SD 1.83 | |
| Notes | |
| – At baseline treated group expelled mean of 3 worms after treatment | |
| – Both groups treated at end: treatment group expelled mean of 4 worms and control group mean of 6 (Table 1) | |
| – Mean changes calculated from the raw data in paper | |
| – No statistics done | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: No: low prevalence of worms; short period of follow‐up | |
Freij et al. (1979 ) (Trial II)
Ascariasis and malnutrition: a study in urban Ethiopian children. American Journal of Clinical Nutrition 21, 1545–1553.
| Design | Randomized controlled trial. |
| Children in a larger morbidity study were tested for Ascaris, then infected children matched with uninfected children. Infected children then randomly allocated to treatment or placebo after being divided up by nutritional status and age | |
| Follow‐up | 34 days |
| Location | Addis Ababa, Ethiopia |
| Age range | 1–4 y; boys only |
| Infection prevalence at baseline | Ascaris lumbricoides: 48.9% |
| Trichuris trichiura: None | |
| Hookworm: None | |
| Treatments | Piperazine (3 g) single dose on 2 consecutive days |
| vs. Placebo | |
| Sample size | Piperazine 24 |
| Placebo 20 | |
| Uninfected 40 | |
| Outcomes | MUAC Weight % standard |
| Findings | |
| – MUAC, cm | |
| Piperazine 14.2 SD 1.3 to 14.6 SD 1.2 | |
| Placebo 14.2 SD 1.2 to 14.5 SD 1.1 | |
| Uninfected 14.2 SD 1.5 to 14.2 SD 1.6 | |
| – Weight, % standard | |
| Piperazine 80.6 SD 10.7 to 80.6 SD 10.7 | |
| Placebo 81.8 SD 9.8 to 82.0 SD 9.3 | |
| Uninfected 79.2 SD 11.7 to 77.8 SD 10.2 | |
| Notes | |
| – No. of expelled worms 7 in piperazine group and 1 in placebo group | |
| – No statistics | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: No: low prevalence of worms; short period of follow‐up | |
Effects on haemoglobin of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 57, 573–579.
| Design | Randomized controlled trial. Double blind. 2 × 2 of micronutrient supplements and helminth treatment. Children randomized to micronutrients then independently randomized to helminth treatment |
| Follow‐up | 8 months |
| Location | Bondo district, Kenya (Lake Victoria) |
| Age range | 8–18 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 14% |
| Trichuris trichiura: 45% | |
| Hookworm: 55% | |
| Schistosoma mansoni: 71% | |
| Treatments | Albendazole 600 mg and praziquantel (40 mg kg−1) and/or micronutrients |
| vs. Placebos | |
| Drugs given in single doses only to children in treatment group with the relevant infections, so not testing mass medication | |
| Micronutrients had 13 vitamins and minerals, including 18 mg of iron; given on schooldays (5 per week) for a school year | |
| Sample size | Micronutrients – placebo 188 |
| Anthelmintics – placebo 187 | |
| Micronutrients – anthelmintics 180 | |
| Placebo – placebo 191 | |
| Outcomes | Haemoglobin change |
| Findings | |
| – Micronutrients – placebo 9.2 g L−1 (95% CI 7.4, 11.1) | |
| Anthelmintics – placebo 8.6 g L−1 (95% CI 6.8, 10.4) | |
| Micronutrients – anthelmintics 10.4 g L−1 (95% CI 8.2, 12.6) | |
| Placebos 5.5 g L−1 (95% CI 3.3, 7.6) | |
| – P = 0.005 (Micronutrients – anthelmintics vs. placebo?) | |
| – Anaemic children had a higher haemoglobin increase than non‐anaemic children in all groups (including placebo) | |
| – There were no interactions between micronutrients and helminth treatment so the effects of each could be assessed in combined groups (Table 2) | |
| – Anthelmintics 9.5 g L−1 (95% CI 8.1, 10.9) n = 279 | |
| No anthelmintics 7.4 g L−1 (95% CI 6.0, 8.9) n = 296 | |
| – Difference of 2.1 g L−1 P = 0.04 | |
| – Regression analysis found that effect of micronutrients on haemoglobin was 3.5 g L−1 (P = 0.0002) and of treatment was 2.0 g L−1 (P = 0.03). No differences by anaemia status | |
| Notes | |
| – At baseline 71% had S. mansoni | |
| – At baseline mean z‐score of weight‐for‐age was −1.1 and mean z‐score of height‐for‐age was −0.96 and mean haemoglobin was 123.7 g L−1 with 41.4% anaemic | |
| – Paper gives 95% CI, not SD | |
| – Same trial as Mwaniki (vitamin A results) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Praziquantel given with albendazole | |
Evaluation of the IMCI guidelines for treatment of intestinal helminth infections among sick children aged 2–4 years in western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 543–548.
| Design | Randomized controlled trial of all sick children at health centres without palmar pallor |
| Follow‐up | 6 months |
| Location | Bungoma district, Kenya |
| Age range | 2–4 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 6% |
| Trichuris trichiura: 1% | |
| Hookworm: 6% | |
| Any infection: 11% | |
| Treatments | Mebendazole 500 mg single dose |
| vs. Placebo | |
| Sample size | All Infected only |
| Mebendazole 166 22 | |
| Placebo 181 20 | |
| Outcomes | Weight gain Z‐score of weight‐for‐age |
| Height gain Z‐score of height‐for‐age | |
| Haemoglobin Z‐score of weight‐for‐height | |
| Findings | |
| – Weight gain 1.19 SD 0.05 kg in placebo | |
| Weight gain 1.21 SD 0.06 kg in mebendazole P = 0.79 | |
| – Height gain 4.17 SD 0.1 cm in placebo | |
| Height gain 4.25 SD 0.11 cm in mebendazole P = 0.57 | |
| – Haemoglobin gain 0.48 SD 0.11 g dL−1 in placebo | |
| Haemoglobin gain 0.54 SD 0.11 g dL−1 in mebendazole P = 0.71 | |
| – Z‐score of weight‐for‐age gain 0.21 SD 0.03 in placebo | |
| Z‐score of weight‐for‐age gain 0.20 SD 0.04 in mebendazole P = 0.87 | |
| – Z‐score of height‐for‐age gain 0.11 SD 0.03 in placebo | |
| Z‐score of height‐for‐age gain 0.14 SD 0.03 in mebendazole P = 0.54 | |
| – Z‐score of weight‐for‐height gain 0.17 SD 0.04 in placebo | |
| Z‐score of weight‐for‐height gain 0.15 SD 0.04 in mebendazole P = 0.75 | |
| – When only children known to be infected analysed, greater mean increase in weight (0.44 kg P = 0.04), height (0.78 cm P = 0.04) and z‐score of weight‐for‐age (0.28 P = 0.02) with mebendazole (Table 3), despite light infections. No difference in haemoglobin | |
| Note | |
| – 11% had at least one infection | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: low prevalence of worms; statistically significant difference between infected treated and uninfected control group before treatment | |
Single dose piperazine therapy for Ascaris lumbricoides: an unsuccessful method of promoting growth American Journal of Clinical Nutrition 34, 2508–2516.
| Design | Randomized controlled trial. Children providing stool samples included. Randomized into treatment and control after stratification for nutritional status |
| Follow‐up | 11 months |
| Location | Bangladesh; village |
| Age range | 1.5–8 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 81% treatment; 80% control |
| Trichuris trichiura: 65% | |
| Hookworm: 5% | |
| Fasciolopsis buski: 40% | |
| Interventions | Piperazine 80 mg kg−1 twice within 2 weeks |
| vs. Placebo | |
| Sample size | Piperazine 74 |
| Placebo 78 At end | |
| Outcomes | % weight‐for‐age |
| % height‐for‐age | |
| % weight‐for‐height | |
| Findings | |
| – Results presented as graphs only | |
| – States that treatment did not significantly affect growth for all children and if analysed by intensity of infection | |
| Notes | |
| – Ascaris mean egg count was 50 eggs g−1 in infected children. 16% of children had a heavy infection (>100 eggs g−1) | |
| – 20% had Giardia lamblia | |
| – Cure rate was 45% 1 month after treatment and 14% 6 months after treatment (Table 4) | |
| – Not used in Cochrane meta‐analysis as graphs only | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no standard deviations | |
Effect of periodic antiascaris and antigiardia treatment on nutritional status of preschool children. American Journal of Clinical Nutrition 36, 79–86.
| Design | Randomized controlled trial. |
| All children in village invited to participate. Randomly divided into 4 groups | |
| Follow‐up | 1 y |
| Location | Guatemala; village |
| Age range | 24–61 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 60% |
| Trichuris trichiura: 8.8% | |
| Hookworm: None | |
| Treatments | 1: Placebo |
| 2: Piperazine 75 mg kg−1 day−1 on 2 consecutive days | |
| 3: Metronidazole 25 mg kg−1 twice a day for 7 days | |
| 4: Piperazine and metronidazole 5 days later | |
| Treated every 2 months | |
| Sample size | Placebo 39 |
| Piperazine 39 | |
| Metronidazole 40 | |
| Both 41 | |
| Outcomes | Weight change Change in % weight‐for‐age |
| Height change Change in % height‐for‐age | |
| Findings | |
| – Weight change, kg | |
| Placebo 1.6324 SD 0.8180 | |
| Piperazine 1.6590 SD 0.7267 | |
| Metronidazole 1.8514 SD 0.6580 | |
| Both 1.9809 SD 0.6713 P = 0.107 | |
| – Height change, cm | |
| Placebo 6.1690 SD 1.7936 | |
| Piperazine 6.0738 SD 2.1159 | |
| Metronidazole 7.0054 SD 2.1138 | |
| Both 6.9762 SD 2.1496 P = 0.064 | |
| – Weight‐for‐age change, % | |
| Placebo 1.5447 SD 3.5503 | |
| Piperazine 1.7468 SD 3.5072 | |
| Metronidazole 2.6705 SD 3.2750 | |
| Both 3.0595 SD 3.7894 P = 0.189 | |
| – Height‐for‐age change, % | |
| Placebo −0.5316 SD 2.6729 | |
| Piperazine −0.2413 SD 1.6090 | |
| Metronidazole 0.1789 SD 2.3319 | |
| Both 0.6682 SD 1.4551 P = 0.069 | |
| Notes | |
| – At baseline prevalence of Giardia was 10–31% across the groups (Table 2) | |
| – Treatment reduced Ascaris prevalence to 34% in both piperazine groups | |
| – At baseline mean % weight‐for‐age ∼71.5; % height‐for‐age ∼85; % weight‐for‐height ∼93% (Table 1) | |
| – Inter‐group differences between placebo and piperazine and metronidazole and both were not significant so they combine the groups in Table 4 into met and no met and show results for all and for younger children – there are significant gains in weight and height (Tables 4,5) | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides. American Journal of Tropical Medicine and Hygiene 59, 791–795.
| Design | Randomized controlled trial. 5 schools randomly assigned to four groups (two placebo schools). A group of Ascaris egg negative children from all schools acted as controls in addition to the placebo group (Fig. 1). Only children with Ascaris included in the four groups. Children with Trichuris egg counts >500 eggs g−1 were excluded |
| Follow‐up | 5 months |
| Location | Koja and Pademangan subdistricts, Indonesia; slums |
| Age range | 6–8 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 58.4% |
| Trichuris trichiura: Some but % not reported; only children with infections <500 eggs g−1 included | |
| Hookworm: Not reported | |
| Interventions | Mebendazole 500 mg single dose |
| vs. Health education every week for 5 months | |
| vs. Mebendazole + health education | |
| vs. Placebo | |
| Sample size | Mebendazole 111 |
| Health education 113 | |
| Mebendazole + health education 74 | |
| Placebo 185 | |
| Outcomes | % weight‐for‐height |
| Findings | |
| – % weight‐for‐height – proportion below cut‐offs | |
| 80–89% 70–79% | |
| Mebendazole 4.5% to 7.2% 10.8% to 10.8% | |
| Health education 0.9% to 0.9% 13.3% to 12.4% | |
| Mebendazole + health education 4.0% to 6.7% 9.4% to 9.4% | |
| Placebo 4.3% to 1.6% 11.9% to 16.2% | |
| – No significant differences between pre‐ and post‐intervention proportions | |
| – No children classified as severely malnourished (<70%) | |
| Notes | |
| – At baseline Ascaris mean egg counts were 1264–2435 eggs g−1 across the four randomized groups, and 88–143 eggs g−1 for Trichuris | |
| – At baseline the sanitary conditions of the children in the mebendazole + education group were worse than the other groups. Also differences across groups for mother's education and egg counts | |
| – Not used in Cochrane meta‐analysis | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Weight‐for‐height expressed as percentage below a threshold | |
Improvements in appetite and growth in helminth‐infected schoolboys three and seven weeks after a single dose of pyrantel pamoate. Parasitology 113, 497–504.
| Design | Randomized controlled trial. Double blind. Boys selected from 3 schools (but does not say how). Allocated at random to treatment or control by descending Ascaris egg count |
| Follow‐up | 7 weeks |
| Location | South Sulawesi, Indonesia; slum |
| Age range | 6–10 y; boys only |
| Infection prevalence at baseline | Ascaris lumbricoides: 89% treatment; 86% control |
| Trichuris trichiura: 100% | |
| Hookworm: Not reported | |
| Treatments | Pyrantel 10 mg kg−1 single dose |
| vs. Placebo | |
| Sample size | Pyrantel 34 |
| Placebo 30 | |
| Outcomes | Weight change Weight‐for‐age change |
| Findings | |
| – Weight change, kg | |
| Pyrantel 0.8 SD 0.9 | |
| Placebo 0.4 SD 0.4, P = 0.02 | |
| – Weight‐for‐age change, % median | |
| Pyrantel 1.5 SD 3.4 | |
| Placebo −0.2 SD 3.3, P = 0.02 | |
| Notes | |
| – At baseline 11% had heavy Ascaris infections (>50 000 eggs g−1) and 11–28% had heavy Trichuris (>10 000 eggs g−1) (Table 1) | |
| – After 7 weeks % Ascaris egg reduction was 82% in pyrantel group and 58% in placebo (arithmetic means); 99% and 64% respectively for geometric means (Table 2) | |
| – At baseline 2/3 below 80% median weight‐for‐age; mean weight‐for‐age ∼74% | |
| – Also presents results at 3 weeks (Table 3). Gain was 0.2 kg more at 3 weeks and 0.4 kg more at 7 weeks | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: No: short period of follow‐up | |
Serum retinol concentrations in children are affected by food sources of β‐carotene, fat intake, and anthelmintic drug treatment. American Journal of Clinical Nutrition 68, 623–629.
| Design | Randomized trial comparing β‐carotene‐rich foods and/or extra fat at a midday meal and deworming. Ascaris‐infected children only. Numbers did not allow complete factorial design so treated as two 2 × 2 studies (Table 1). No deworming alone group |
| Follow‐up | 21 days |
| Location | West Sumatra, Indonesia |
| Age range | 3–6 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 100% |
| Trichuris trichiura: 18% | |
| Hookworm: 8% | |
| Treatments | Group O: Basic meal |
| Group FD: Basic meal + fat + levamisole | |
| Group C: Basic meal + β‐carotene | |
| Group CFD: Basic meal + β‐carotene + fat + levamisole | |
| Group CD: Basic meal + β‐carotene + levamisole | |
| Group CF: Basic meal + β‐carotene + fat | |
| Food provided for 21 days, 6 days per week; used sweet potato and coconut oil and milk | |
| Deworming was done 1 week before meals started; single dose, ? dose size | |
| Sample size | Group O: 41 Group CFD: 42 |
| Group FD: 42 Group CD: 43 | |
| Group C: 41 Group CF: 43 | |
| Outcomes | Serum retinol change |
| Findings | |
| – Ascaris egg counts and serum retinol negatively correlated at baseline P < 0.04 | |
| – Change – low baseline egg count (<3200 eggs g−1) | |
| O: 0.06 SE 0.04 n22 | |
| FD: 0.12 SE 0.08 n17 | |
| C: 0.24 SE 0.07 n17 | |
| CFD: 0.23 SE 0.07 n20 | |
| CD: 0.21 SE 0.07 n17 | |
| CF: 0.54 SE 0.10 n20 | |
| – Change – high baseline egg count (>3200 eggs g−1) | |
| O: 0.00 SE 0.09 n17 | |
| FD: 0.22 SE 0.07 n22 | |
| C: 0.20 SE 0.05 n19 | |
| CFD: 0.46 SE 0.07 n20 | |
| CD: 0.37 SE 0.07 n21 | |
| CF: 0.23 SE 0.06 n19 | |
| – When groups O, FD, C and CFD are compared, anova showed that fat and levamisole benefited children with high egg counts P = 0.05 (page 626) | |
| – When groups C, CD, CF and CFD are compared, anova showed that the addition of levamisole benefited children with high egg counts most P < 0.001 (page 627) | |
| Notes | |
| – Mean Ascaris egg count at baseline 5003–6078 across groups | |
| – Levamisole cure rate was 84–94% (Table 3) | |
| – At baseline % height‐for‐age ∼91; % weight‐for‐age ∼78% | |
| – Mean serum retinol <0.7 µmol in all groups at baseline and 11% had levels <0.35 µmol | |
| – Note the short duration | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: subjects given a meal in addition to levamisole; no untreated controls; short period of follow‐up | |
A randomized controlled trial of the effect of antihelminthic treatment and micronutrient fortification on health status and school performance of rural primary school children. Annals of Tropical Paediatrics 21, 319–333.
| Design | Randomized controlled trial. |
| Double‐blind study in 11/72 randomly selected schools. Children individually randomized into 6 groups. Testing deworming and biscuits fortified with vitamin A, iron and micronutrients | |
| Follow‐up | 16 weeks |
| Location | KwaZulu‐Natal, South Africa |
| Age range | 8–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 28.8% dewormed; 26.4% not dewormed |
| Trichuris trichiura: 53.7% dewormed; 54.5% not dewormed | |
| Hookworm: 3.1% dewormed; 3.5% not dewormed | |
| Schistosoma mansoni: 24.5% dewormed; 24.6% not dewormed | |
| Treatments | 1: Albendazole + praziquantel, vitamin A‐50% RDA, iron, micronutrients |
| 2: Albendazole + praziquantel, vitamin A‐100% RDA | |
| 3: Albendazole + praziquantel, unfortified biscuit | |
| 4: Placebo, vitamin A‐50% RDA, iron, micronutrients | |
| 5: Placebo, vitamin A‐100% RDA | |
| 6: Placebo, unfortified biscuit | |
| Single dose, 400 mg of albendazole, 40 mg kg−1 praziquantel | |
| Micronutrient content (page 321); biscuits given 5 days per week for 16 weeks | |
| Sample size | Varies a lot for the different outcomes. Total n = 579. These are for serum retinol (Table V) |
| 1: 41 4: 54 | |
| 2: 52 5: 40 | |
| 3: 43 6: 67 | |
| Outcomes | Serum retinol |
| Findings | |
| – Group 2 – vitamin A deficiency fell from 32.1% to 15.1% P = 0.067; differences in other groups NS (Table V) | |
| – Mean serum retinol (Table V): | |
| Rose in Group 2: 0.84 SD 10.44 to 0.95 SD 8.16 P < 0.05 | |
| Fell in Group 3: 0.89 SD 12.27 to 0.77 SD 9.02 P < 0.05 | |
| – Anaemia prevalence stayed fairly constant throughout | |
| – States that treatment had no significant effect on weight, height, z‐score of height‐for‐age or z‐score of weight‐for‐age; does not show data (page 324) | |
| – States that no significant changes occurred in haematological variables in the 6 groups; does not show data (page 324). Also no effect if only infected children are analysed | |
| – In Discussion says that significant changes in vitamin A status of dewormed children given vitamin A (groups 1 and 2) vs. those not dewormed (groups 4 and 5) (page 330) | |
| Notes | |
| – Deworming significantly reduced the prevalence of all the worms, although prevalence of Trichuris remained 40% (mostly light, <20 eggs per coverslip) (Table II) | |
| – At baseline 7.3% stunted, 0.8% underweight, 15.5% anaemic, 34.4% vitamin A deficient (<0.7 µmol L−1) | |
| – Data on weight, height and haemoglobin were not presented | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: praziquantel given with albendazole, and treatments given with micronutrient supplements or food, so no useful comparisons | |
Epidemiology of helminth infections: implications for parasite control programmes, a South African perspective. Public Health Nutrition 4, 1211–1219.
| Design | Randomized controlled trial. |
| 11 of 72 randomly selected schools. Children individually randomized into treatment and control groups | |
| Follow‐up | 16 weeks |
| Location | KwaZulu‐Natal, South Africa |
| Age range | 8–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 29.5% treated; 28.1% untreated |
| Trichuris trichiura: 51.9% treated; 54.0% untreated | |
| Hookworm: 3.1% treated; 2.9% untreated | |
| Schistosoma mansoni: 0.8% treated; 0.7% untreated | |
| Schistosoma haematobium: 22.3% treated; 21.9% untreated | |
| Treatments | Albendazole (400 mg) + praziquantel (40 mg kg−1) |
| vs. Placebo | |
| Single dose | |
| Sample size | Wt – baseline Wt – 16 weeks Ht –baseline Ht – 16 weeks |
| Albendazole + 127 124 129 124 | |
| praziquantel | |
| Placebo 136 126 137 126 | |
| Outcomes | Weight % <−2 z‐score of weight‐for‐age |
| Height % <−2 z‐score of height‐for‐age | |
| Findings | |
| – Anthropometry did not vary across groups or time periods (Table 2) | |
| – Mean weight treated group: 27.1 SD 3.6 kg to 28.5 SD 4.0 | |
| Mean weight control group: 26.8 SD 3.5 kg to 28.0 SD 3.6 | |
| – Mean height treated group: 127.8 SD 6.9 cm to 129.4 SD 6.8 | |
| Mean height control group: 127.3 SD 5.0 cm to 129.1 SD 5.4 | |
| Notes | |
| – Presumably these are some of the same children as the other 2001a, 2001b) paper? | |
| – Deworming significantly reduced the prevalence of Ascaris, Trichuris and S. haematobium | |
| – At baseline only ∼8% stunted, 0.8% underweight | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: praziquantel given with albendazole; very low prevalence of underweight and stunting | |
Anthelminthic treatment raises plasma iron levels but does not decrease the acute‐phase response in Jakarta school children. Southeast Asian Journal Tropical Medicine Public Health 27, 742–753.
| Design | Randomized controlled trial. Double blind. Children selected from 2 schools but does not say how were chosen. Only infected children entered into trial. Selected 160 with highest intensity infections and divided into treatment and control on matching egg counts |
| Follow‐up | 10 days |
| Location | North Jakarta, Indonesia |
| Age range | 8–11 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 81.6% |
| Trichuris trichiura: 88.3% | |
| Hookworm: None | |
| Treatments | Albendazole 400 mg |
| vs. Placebo | |
| Single dose | |
| Sample size | Albendazole 40 |
| Placebo 35 (for the haemoglobin results – Table 3) | |
| Outcomes | Haemoglobin change |
| Findings | |
| – Albendazole +0.73 SD 2.7 g dL−1 | |
| Placebo −0.15 SD 2.54 g dL−1 | |
| – Changes NS | |
| – Also gives plasma iron values (Table 3) – increased significantly in both groups but more so in the albendazole group (page 746) | |
| Notes | |
| – Geometric mean egg count ∼75 eggs g−1 Ascaris and ∼24 eggs g−1 Trichuris (Table 2) | |
| – Deworming reduced Ascaris prevalence to 0% and Trichuris to 46% (Table 1) | |
| – At baseline 19.2% stunted, 24.2% underweight, 6.7% wasted, 30% anaemic | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: short period of follow‐up | |
Ascaris and malnutrition in a group of Brazilian children – a follow‐up study. Journal of Topical Pediatrics 28, 41–43.
| Design | Randomized controlled trial. |
| All children of 9 villages included and randomly allocated to treatment or placebo | |
| Follow‐up | 10 months |
| Location | Sao Paulo state, Brazil; rural |
| Age range | 1–8 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 64% treatment; 53% controls |
| Trichuris trichiura: 26% treatment; 20% controls | |
| Hookworm: 12% treatment; 8% controls | |
| Interventions | Mebendazole 100 mg twice a day for 3 days |
| vs. Placebo | |
| Sample size | Mebendazole 165 |
| Placebo 172 | |
| Outcomes | Weight‐for‐age |
| Findings | |
| – 51% of treated children improved their reference weight‐for‐age vs. 49% controls | |
| 35% of treated children decreased their reference weight‐for‐age vs. 33% controls | |
| 14% of treated children had no change vs. 18% controls | |
| – No significant differences if analysed by change in egg counts (Table III) | |
| Notes | |
| – At baseline overall prevalence of Ascaris was 59%, Trichuris 22%, hookworm 9% and Giardia 32%. 10% had heavy Ascaris infection (not defined) | |
| – At 4 months prevalence of Ascaris was 51%, Trichuris 4%, hookworm 1% and Giardia 15% in treated children. At 10 months prevalence of Ascaris was 52%, Trichuris 11%, hookworm 8% and Giardia 24% in treated children | |
| – Not used in the Cochrane meta‐analysis as results reported as % improved | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No; data given as percentage improved in terms of weight‐for‐age | |
Effects of albendazole on growth of primary school children and the prevalence and intensity of soil‐transmitted helminths in Sierra Leone. Journal of Topical Pediatrics 42, 371–372.
| Design | Randomized controlled trial |
| Follow‐up | 6 months |
| Location | Sierra Leone; urban and rural |
| Age range | 6–10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 32% urban; 26% rural |
| Trichuris trichiura: 65% urban; 1% rural | |
| Hookworm: 10% urban; 25% rural | |
| Strongyloides stercoralis: 6% urban; 14% rural | |
| Interventions | Albendazole 400 mg |
| vs. Placebo (no treatment?) | |
| Sample size | Urban Rural |
| Albendazole 71 68 | |
| Placebo 24 24 | |
| Outcomes | Z‐score of weight‐for‐age change |
| Z‐score of height‐for‐age change | |
| Z‐score of weight‐for‐height change | |
| Findings (± SEM?) | |
| – Change in z‐score of weight‐for‐age | |
| Urban Rural | |
| Albendazole 1.02 (0.09) 1.04 (0.03) | |
| Placebo −0.05 (0.17) −0.10 (0.21) | |
| – Change in z‐score of height‐for‐age | |
| Urban Rural | |
| Albendazole 1.01 (0.02) 0.83 (0.03) | |
| Placebo −0.16 (0.13) −0.10 (0.03) | |
| – Change in z‐score of weight‐for‐height | |
| Urban Rural | |
| Albendazole 1.04 (0.07) 0.28 (0.17) | |
| Placebo −0.03 (0.03) −0.07 (0.27) | |
| – All treated groups showed significant improvements over baseline; control groups showed no change from baseline (Table 2) | |
| Notes | |
| – Prevalence fell significantly after treatment except hookworm in the urban group and Trichuris in the rural group | |
| – At baseline mean z‐score of weight‐for‐age −2.25 to −1.07; mean z‐score of height‐for‐age −2.27 to −1.14; mean z‐score of weight‐for‐height −1.73 to −0.67 | |
| – Not used in the Cochrane meta‐analysis as z‐scores only | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Effects of iron fortification in a school feeding scheme and anthelmintic therapy on the iron status and growth of six‐ to eight‐year‐old schoolchildren. Food and Nutrition Bulletin 17, 11–21.
| Design | Randomized controlled trial. Children randomly selected from 5 schools. Schools allocated to iron un/fortified soup (how?) then pupils within schools randomly allocated to treatment or placebo |
| Follow‐up | 11 months |
| Location | Worcester Region, South Africa; urban and rural |
| Age range | 6–8 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 20.0% |
| Trichuris trichiura: 38.1% | |
| Hookworm: None | |
| Hymenolepis nana: 20.7% | |
| Any worm species: 58.7% | |
| Treatments | FeA: iron fortified soup + albendazole vs. |
| FeP: iron fortified soup + placebo vs. | |
| BA: Unfortified soup + albendazole vs. | |
| BP: Unfortified soup + placebo | |
| Albendazole given twice, 4 months apart, 400 mg | |
| Soup given every school day for 6 months; also had vitamin C added | |
| Sample size | Low baseline iron Adequate baseline iron |
| FeA 20 30 | |
| FeP 24 30 | |
| BA 15 22 | |
| BP 14 23 | |
| Outcomes | Haemoglobin change Z‐score of weight‐for‐height change |
| Weight change Z‐score of weight‐for‐age change | |
| Height change Z‐score of height‐for‐age change | |
| Findings | |
| Haemoglobin change, g dL−1 | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 0.7 SD 0.7 0.5 SD 0.7 | |
| FeP 0.2 SD 0.6 0.4 SD 0.6 | |
| BA 0.3 SD 0.6 0.2 SD 0.6 | |
| BP 0.2 SD 0.6 0.3 SD 0.8 | |
| Wt change, kg | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 2.8 SD 1.1 2.9 SD 1.1 | |
| FeP 2.3 SD 0.7 2.6 SD 0.8 | |
| BA 2.5 SD 0.7 2.2 SD 0.6 | |
| BP 2.2 SD 0.6 3.0 SD 1.6 | |
| Ht change, cm | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 6.1 SD 1.2 5.8 SD 0.9 | |
| FeP 5.6 SD 1.2 5.8 SD 1.0 | |
| BA 5.8 SD 0.9 5.5 SD 0.9 | |
| BP 5.1 SD 1.1 5.8 SD 0.8 | |
| Z‐score of weight‐for‐age change | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 0.22 SD 0.23 0.15 SD 0.17 | |
| FeP 0.07 SD 0.23 0.06 SD 0.23 | |
| BA 0.09 SD 0.24 0.02 SD 0.22 | |
| BP 0.12 SD 0.17 0.19 SD 0.34 | |
| Z‐score of height‐for‐age change | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 0.13 SD 0.21 0.06 SD 0.13 | |
| FeP 0.06 SD 0.23 0.05 SD 0.19 | |
| BA 0.09 SD 0.20 0.03 SD 0.16 | |
| BP −0.03 SD 0.15 0.08 SD 0.12 | |
| Z‐score of weight‐for‐height change | |
| Low iron at baseline Adequate iron at baseline | |
| FeA 0.25 SD 0.38 0.21 SD 0.29 | |
| FeP 0.15 SD 0.29 0.12 SD 0.30 | |
| BA 0.10 SD 0.26 0.08 SD 0.28 | |
| BP 0.25 SD 0.25 0.25 SD 0.44 | |
| – Treatment was associated with positive changes in haemoglobin compared with placebo in children with low iron stores, P = 0.0393 | |
| – Treatment was associated with greater increases in height (P = 0.0344) and weight (P = 0.0346) at 11 months in children with low iron at baseline | |
| – In children with adequate iron stores, neither treatment or fortification alone affected anthropometric outcomes. There was significant interaction between the interventions at 11 months P = 0.0115 | |
| Notes | |
| – At baseline 42.5% were anaemic according to WHO thresholds; 18% were stunted and 14% were underweight | |
| – Ascaris prevalence showed significant treatment effects – fell during the intervention but rose again by 11 months | |
| – Also gives results at 5 months | |
| – Other iron outcomes are MCV, MCH, Ferritin and TIBC and TS% | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: No – albendazole given with food, no untreated controls; prevalence of intestinal nematodes probably <50% | |
Ascaris and Trichuris do not contribute to growth retardation in primary school children. Southeast Asian Journal of Tropical Medicine and Public Health 26, 322–328.
| Design | Randomized controlled trial. Pupils from 16 schools who provided a stool sample randomized to treatment or control. Block randomization by school, sex, egg count and weight. Single blind |
| Follow‐up | 2 y |
| Location | Kuala Lumpur and Selangor, Malaysia; urban and peri‐urban |
| Age range | 8 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 67% treatment; 66% control |
| Trichuris trichiura: 66% treatments; 69% control | |
| Hookworm: 6% treatment; 5% control | |
| Interventions | Mebendazole 100 mg + pyrantel 100 mg every 3 months |
| vs. Placebo | |
| Sample size | Boys Girls |
| Treatment 66 95 | |
| Placebo 59 86 See note below | |
| Outcomes | Rate below −2 SD for weight‐for‐age |
| Rate below −2 SD for height‐for‐age | |
| Rate below −2 SD for weight‐for‐height | |
| Findings | |
| Weight‐for‐age rate below −2 SD, % | |
| Boys Girls | |
| Treatment 31.2 to 27.3 22.9 to 20.0 | |
| Placebo 40.9 to 25.4 21.8 to 24.4 | |
| Height‐for‐age rate below −2 SD, % | |
| Boys Girls | |
| Treatment 15.6 to 10.3 15.6 to 10.5 | |
| Placebo 22.7 to 20.3 8.9 to 5.8 | |
| Weight‐for‐height rate below −2 SD, % | |
| Boys Girls | |
| Treatment 13.0 to 18.2 11.9 to 9.2 | |
| Placebo 13.6 to 16.9 20.8 to 17.2 | |
| – No significant differences between final and initial proportions | |
| – 13.5% of girls excluded from weight‐for‐height measurements | |
| Notes | |
| – Gives initial and final weights and heights (Table 2a), but the data are not paired. It is not clear that this does not also apply to the data given above | |
| – Not used in the Cochrane meta‐analysis as no standard deviations | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – no standard deviations and data given as percentage subjects with weight‐for‐age, height‐for‐age and weight‐for‐height less than −2 SD | |
Raj et al. (1998a )
Intestinal geohelminthiasis and growth in pre‐adolescent primary school children in northeastern peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 29, 112–117.
| Design | 100 children from one school divided into infected and uninfected. All infected treated; uninfected as controls. Does not say how 100 selected |
| Follow‐up | 1 y |
| Location | Kelantan, Malaysia; town |
| Age range | 8–9 y |
| Infection prevalence at baseline | Ascaris lumbricoides and/or T. trichiura: 73% |
| Hookworm: Not found | |
| Treatments | Albendazole 400 mg |
| vs. No treatment | |
| Treated at baseline, 6 and 9 months | |
| Sample size | Albendazole 73 |
| Controls 27 | |
| Outcomes | Weight change Weight‐for‐age % median change |
| Height change Height‐for‐age % median change | |
| Weight‐for‐height % median change | |
| Findings | |
| – Weight change, kg | |
| Albendazole 2.8 (1.0) (median change and Q dev’n Table 2 – note b) | |
| Control 2.5 (0.5) | |
| – Height change, cm | |
| Albendazole 5.4 (0.9) (median change and Q dev’n Table 2 – note b) | |
| Control 5.2 (0.5) | |
| – Weight‐for‐age, change in % median | |
| Albendazole 0.1 (2.3) (median change and Q dev’n Table 2 – note b) | |
| Control −0.6 (1.9) | |
| – Height‐for‐age, change in % median | |
| Albendazole 0.3 (0.6) (median change and Q dev’n Table 2 – note b) | |
| Control 0.3 (0.5) | |
| – Weight‐for‐height, change in % median | |
| Albendazole 1.0 (3.4) (median change and Q dev’n Table 2 – note b) | |
| Control −0.1 (2.4) | |
| – All NS P > 0.1, treated vs. uninfected at each time point | |
| – Height and weight improved more in girls (Table 3) | |
| Notes | |
| – Groups comparable about age and gender, but no information on any other socio‐economic variables; no significant differences for baseline anthropometric (Table 2) | |
| – 16/73 had >50 000 eggs g−1 Ascaris | |
| – Deworming reduced Ascaris prevalence to 16% and Trichuris to 54% (Table 1); by the end 35% of controls had Ascaris and 23% had Trichuris | |
| – 20% of all children <90% height‐for‐age | |
| Says follow‐up not done on 2 children, but does not say which group they are in | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – not randomized; gives medians, not means so no standard deviations | |
Mahendra Raj et al. (1998b )
Ascariasis, trichuriasis, and growth of schoolchildren in northeastern peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 29, 729–734.
| Design | Children from one class at a school tested for worms. Infected were treated and uninfected acted as controls |
| Follow‐up | 14 weeks |
| Location | Kota Bharu town, Malaysia |
| Age range | Mean age 8.2 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 74.6% |
| Trichuris trichiura: 85.9% | |
| Hookworm: None | |
| Treatments | Albendazole 400 mg two doses a week apart vs. No treatment |
| Sample size | Albendazole 71 |
| Controls 32 | |
| Outcomes | Weight gain Z‐score of weight‐for‐age gain |
| Height gain Z‐score of height‐for‐age gain | |
| Z‐score of weight‐for‐height gain | |
| Findings | |
| – Median weight gain in treated children 0.6 kg (0.5 quartile deviation) | |
| Median weight gain in control children 0.2 kg (0.4) | |
| P = 0.008 | |
| – Median height gain in treated children 2.0 cm (0.4 quartile deviation) | |
| Median height gain in control children 1.7 cm (0.5) | |
| P = 0.07 | |
| – Median z‐score of weight‐for‐age gain in treated children −0.04 (0.11 quartile deviation) | |
| Median z‐score of weight‐for‐age gain in control children −0.14 (0.09) | |
| P = 0.003 | |
| – Median z‐score of height‐for‐age gain in treated children 0.10 (0.10 quartile deviation) | |
| Median z‐score of height‐for‐age gain in control children 0.02 (0.08) | |
| P = 0.03 | |
| – Median z‐score of weight‐for‐height gain in treated children −0.15 (0.20 quartile deviation) | |
| Median z‐score of weight‐for‐height gain in control children −0.20 (0.19) | |
| P = 0.03 | |
| – States treatment associated with gains in WAZ and weight‐for‐height independent of social class (Table 3 and page 730) | |
| Notes | |
| – Two groups were significantly different in social class (Table 1) | |
| – Treatment reduced prevalence to 39% Ascaris, 79% Trichuris; by the end controls had 7% Ascaris, 38% Trichuris; median egg count in infected children fell from 19.6 × 103 to 0 Ascaris and 2.8 × 103 to 1.6 × 103 Trichuris (Table 1) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – not randomized; no infected control; gives medians, not means so no standard deviations | |
Influence of enteral parasites on the blood vitamin A levels in preschool children orally supplemented with retinol and/or zinc. European Journal of Clinical Nutrition 68, 623–629.
| Design | Children with Ascaris and/or G. lamblia randomly selected. Half treated (randomly selected?). These two groups each randomized to vitamin and/or zinc or placebo (Fig. 1) |
| Follow‐up | 30 days |
| Location | Manaus, Brazil |
| Age range | 3–7 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 47.8% |
| Trichuris trichiura: 66.6% | |
| Hookworm: 8.0% | |
| Other intestinal nematodes: 2.4%; any intestinal nematode: 53%; Giardia lamblia: 29.3% | |
| Treatments | 1: Mebendazole + metronidazole, zinc, vitamin A |
| 2: Zinc, vitamin A | |
| 3: Mebendazole + metronidazole, zinc | |
| 4: Zinc | |
| 5: Mebendazole + metronidazole, vitamin A | |
| 6: Vitamin A | |
| 7: Mebendazole + metronidazole | |
| 8: Placebos for zinc and vitamin A | |
| Mebendazole 50 mg twice a day for 3 days. No treatment placebos? | |
| Vitamin A and zinc supplements daily | |
| Sample size | 30 per group; all had 15 males |
| Outcomes | Serum retinol change |
| Findings | |
| – Serum retinol change, µg dL−1 | |
| Treated groups Untreated groups | |
| 1 male: 6.7 2 males: −0.4 | |
| 1 female: 7.0 2 females: 0.4 | |
| 3 males: 4.7 4 males: −0.5 | |
| 3 females: 5.5 4 females: 0.3 | |
| 5 males: 9.7 6 males: 1.2 | |
| 5 females: 7.3 6 females: 0.0 | |
| 7 males: 5.0 8 males: −1.0 | |
| 7 females: 3.8 8 females: −2.2 | |
| – No SD given for the changes, but baseline and final means SD. SD given in Table 6 | |
| – All treated group differences are significantly different from baseline P < 0.05; no significant increases in the untreated groups | |
| Notes At baseline 12% stunted | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: experimental groups given metronidazole (a broad spectrum antimicrobial) as well as mebendazole | |
Michaelson et al. (1985 )
Hookworm infection in Kweneng District, Botswana. A prevalence survey and a controlled treatment trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 79, 848–851.
| Design | Randomized controlled trial. One school with high hookworm prevalence selected and children randomly allocated to treatment or placebo |
| Follow‐up | 5 months |
| Location | Kweneng District, Botswana |
| Age range | 5–14 y |
| Infection prevalence at baseline | Ascaris lumbricoides: None |
| Trichuris trichiura: None | |
| Hookworm: 86–90% in four districts | |
| Interventions | Tetrachloroethylene 0.1 mL kg−1 single dose |
| vs. Placebo (cough mixture) | |
| Sample size | Haemoglobin Weight‐for‐height |
| Treatment 83 63 | |
| Placebo 78 58 | |
| Outcomes | Haemoglobin change % weight‐for‐height change |
| Findings | |
| – Haemoglobin change, g dL−1 | |
| Tetrachloroethylene 0.22 | |
| Placebo 0.09 NS | |
| – % weight‐for‐height change | |
| Tetrachloroethylene −1.3 | |
| Placebo −0.4 NS | |
| Notes | |
| – 99% of hookworm infections had egg counts below 2000 eggs g−1. | |
| – Treatment significantly reduced the number of children excreting eggs and the egg output of children still infected | |
| – No standard deviations given for outcomes | |
| – Not used in the Cochrane meta‐analysis | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – no standard deviations | |
Effects on serum retinol of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 56, 666–673.
| Design | Randomized controlled trial. Double blind. 2 × 2 of micronutrient supplements with placebo and helminth treatment with placebo. Children randomized to micronutrients then independently randomized to helminth treatment |
| Follow‐up | 8 months |
| Location | Bondo district, Kenya (Lake Victoria) |
| Age range | 9–18 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 55–66% in four study groups |
| Trichuris trichiura: 42–49% in four study groups | |
| Hookworm: 51–60% in four study groups | |
| Schistosoma mansoni: 69–74% in four study group | |
| Treatments | Albendazole 600 mg and praziquantel 40 mg kg−1 and/or micronutrients |
| vs. Placebos | |
| Drugs given in single doses only to children in treatment group with the relevant infections, so not testing mass medication | |
| Micronutrients had 13 vitamins and minerals, including 1000 µg of vitamin A | |
| Sample size | Groups combined in analysis (Table 2): |
| No anthelmintics 257 No micronutrients 238 | |
| Anthelmintics 239 Micronutrients 258 | |
| Outcomes | Serum retinol increase |
| Findings | |
| – No anthelmintics 0.26 µmol (0.21, 0.31) | |
| Anthelmintics 0.29 µmol (0.24, 0.34) | |
| – P = 0.38 | |
| – There were no interactions between micronutrients and helminth treatment so the effects of each could be assessed in combined groups (Table 2) | |
| – Supplementation predicted serum retinol at 8 months, but treatment did not P = 0.18 (Table 3), except in children with S. mansoni infection P = 0.01 | |
| Notes | |
| – At baseline mean z‐score of weight‐for‐age was −1.11 and mean z‐score of height‐for‐age was −0.96 and mean serum retinol was 0.94 µmol | |
| – Paper gives 95% CI, not SD | |
| – Same trial as Freij et al. (1979) (haemoglobin results) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – treatments were praziquantel and albendazole, with or without micronutrients | |
Northrop‐Clewes et al. (2001 )
Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. American Journal of Clinical Nutrition 73, 53–60.
| Design | Randomized, double blind, controlled trial. |
| 8 villages allocated randomly to treatment and control. Children randomly selected from villages, regardless of infection status | |
| Follow‐up | 1 y |
| Location | Jamalpur, Bangladesh |
| Age range | 2–6 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 73% treatment; 71% control |
| Trichuris trichiura: 65% treatment; 16% control | |
| Hookworm: 4% treatment; 11% control | |
| Treatments | Pyrantel 100 mg single dose then mebendazole 500 mg every two months |
| vs. Placebo | |
| Sample size | MUAC WAZ HAZ |
| Pyrantel– mebendazole 49 46 43 | |
| Placebo 48 43 42 | |
| Outcomes | MUAC Z‐score of weight‐for‐age |
| Z‐score of height‐for‐age | |
| Findings (mean SD) | |
| – MUAC, cm | |
| Treated children 15.1 SD 0.13 to 15.3 SD 0.13 | |
| Placebo children 14.8 SD 0.13 to 14.9 SD 0.14 | |
| – Z‐score of weight‐for‐age | |
| Treated children −2.44 SD 0.11 to −2.27 SD 0.12 | |
| Placebo children −2.69 SD 0.12 to −2.39 SD 0.11 | |
| – Z‐score of height‐for‐age | |
| Treated children −2.31 SD 0.21 to −2.28 SD 0.18 | |
| Placebo children −3.03 SD 0.21 to −2.89 SD 0.18 | |
| – Significant group effect for z‐score of height‐for‐age, P = 0.016 – so treatment children taller throughout | |
| – Group × time interaction MUAC, P = 0.01 – placebos worsened at months 2 and 4 | |
| But no interactions for z‐score of weight‐for‐age and z‐score of height‐for‐age | |
| – ancova showed treatment had no effect on change in weight‐for‐age, height‐for‐age and weight‐for‐height at 8 and 12 months (page 56) | |
| Notes | |
| – Also gives means at months 2, 4, 6 and 8 (Table 2) | |
| – At baseline mean eggs g−1 in infected children 1007 eggs g−1 Ascaris, 121 eggs g−1 Trichuris, 66 eggs g−1 hookworm | |
| By 12 months treatment reduced Ascaris to 4%, Trichuris to 10%, hookworm to 0% | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes, though small sample size of clusters | |
Double‐blind placebo‐controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. Journal of Infectious Diseases 179, 996–1003.
| Design | Block randomized controlled trial; double blind. |
| Children in each site randomized to 4 groups | |
| Follow‐up | 6 months |
| Locations | Leyte, Philippines |
| Sichuan Province, China | |
| Kisumu Province, Kenya | |
| Kwale District, Kenya | |
| Age range | 6–17 y Philippines |
| 5–16 y China | |
| 6–19 y Kisumu | |
| 4–18 y Kwale | |
| Infection prevalence at baseline | Ascaris lumbricoides: 60.5% (praziquantel + albendazole); 57.2% (praziquantel); 56.6% (albendazole); 60.2% (placebo) |
| Trichuris trichiura: 81.8% (praziquantel + albendazole); 79.4% (praziquantel); 76.8% (albendazole); 81.0% (placebo) | |
| Hookworm: 51.7% (praziquantel + albendazole); 48.2% (praziquantel); 44.4% (albendazole); 52.1% (placebo) | |
| Schistosoma japonicum (Philippines and China): 44.8–59.3% | |
| Schistosoma mansoni (Kisumu, Kenya): 71.6–84.0% | |
| Schistosoma haematobium (Kwale, Kenya): 80.4–89.7% | |
| Treatments | Albendazole 400 mg and/or praziquantel 1 × 40 mg kg−1 or 2 × 30 mg kg−1 |
| vs. Placebos | |
| Sample size | Albendazole + praziquantel 392 |
| Albendazole 387 | |
| Praziquantel 380 | |
| Placebos 381 | |
| 90% followed up at 6 months | |
| Outcomes | Haemoglobin |
| Weight | |
| Height | |
| MUAC | |
| Triceps skinfold thickness | |
| Findings | |
| – Mean haemoglobin increase in praziquantel‐treated children = 0.271 SD 1.802 g dL−1 | |
| – In placebo children = 0.017 SD 1.938 g dL−1 | |
| – Significance? Does it mean praziquantel alone praziquantel and praziquantel + albendazole combined? (page 1000) | |
| – Says no significant improvement seen after albendazole treatment (page 1000) | |
| – Says no significant differences seen in anthropometric measurements (page 1000) | |
| Notes | |
| – Main aim was testing cure rates and side effects of the 2 drugs in combination | |
| – At baseline mean haemoglobin was ∼11.9 g dL−1 | |
| – Comments on how slowly haemoglobin levels improve after praziquantel treatment in the absence of supplemental iron – after a year children infected with Schistosoma still catching up with uninfected children (page 1000) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No, data only given on haemoglobin change only; cannot determine sample size treated with albendazole only | |
Effective community intervention to improve hemoglobin status in preschoolers receiving once‐weekly iron supplementation. American Journal of Clinical Nutrition 65, 1057–1061.
| Design | Randomized controlled trial. |
| Iron supplements with or without worming on iron status. Double blind. | |
| No worming only group. All children included | |
| Follow‐up | 9 weeks |
| Location | West Javanese village, Indonesia |
| Age range | 2–5 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 55.5% |
| Trichuris trichiura: 29.3% | |
| Hookworm: 0% | |
| Treatments | Weekly iron and albendazole 400 mg single dose |
| vs. Weekly iron and placebo | |
| vs. Placebos | |
| Sample size | Iron and albendazole 95 |
| Iron 96 | |
| Placebos 98 | |
| Outcomes | Haemoglobin change Z‐score of height‐for‐age change |
| Weight gain Z‐score of weight‐for‐age change | |
| Height gain Z‐score of weight‐for‐height change | |
| Findings | |
| – Haemoglobin change, g L−1 | |
| Iron + albendazole 7.5 SD 8.8 | |
| Iron 6.4 SD 10.6 | |
| Placebos 1.9 SD 8.0 | |
| – Both iron + albendazole and iron greater than placebo, P < 0.05, but not different from each other P = 0.53 | |
| – Significant (P = 0.001) negative correlation between haemoglobin at baseline and change in haemoglobin in the treatment groups, so lowest haemoglobin saw largest increases | |
| – Significant decreases in the prevalence of anaemia in both treatment groups | |
| Iron + albendazole Iron Placebos | |
| Weight gain 0.51 SD 0.73 0.45 SD 0.58 0.34 SD 0.63 | |
| Height gain 1.20 SD 0.9 1.40 SD 1.00 1.20 SD 1.0 | |
| Z‐score of height‐for‐age gain 0.03 SD 0.24 0.07 SD 0.27 0.03 SD 0.26 | |
| Z‐score of weight‐for‐age gain 0.16 SD 0.46 0.14 SD 0.36 0.06 SD 0.41 | |
| Z‐score of weight‐for‐height gain 0.20 SD 0.65 0.12 SD 0.49 0.03 SD 0.56 | |
| – No between‐group differences in changes in weight, height, weight‐for‐age, height‐for‐age, weight‐for‐height | |
| But weight‐for‐age and weight‐for‐height both significantly improved within the treatment groups but not the placebo group; and height‐for‐age improved significantly within the iron group (Table 3) | |
| Notes | |
| – Median egg count in infected children 1245 eggs g−1 Ascaris and 38 eggs g−1 Trichuris | |
| – Albendazole reduced Ascaris prevalence to 2.2%, Trichuris to 16.1% and hookworm 0 | |
| – At baseline 40.1% stunted, 39.4% underweight and 6.9% wasted | |
| – No additional effect of albendazole above iron, but there was not any hookworm | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no group given only albendazole, micronutrient supplements given as well | |
Partnership for Child Development (unpublished)
A randomised trial of six‐monthly deworming on the growth and educational achievements of Vietnamese school children. Unpublished.
| Design | Cluster randomized controlled trial. |
| 80 schools randomized to treatment (n = 40) or placebo (n = 40). All children in year 3 included; if >40 then 40 were randomly selected | |
| Follow‐up | 2 y |
| Location | Vietnam |
| Age range | Mean 104.5 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 70.7% |
| Trichuris trichiura: 83.6% | |
| Hookworm: 7.2% | |
| Treatments | Albendazole 400 mg every 6 months |
| vs. Placebo (first treatment) then nothing | |
| Sample size | Albendazole 1341 |
| Placebo 1318 | |
| Outcomes | Weight gain |
| Height gain | |
| MUAC gain | |
| Triceps gain | |
| Findings | |
| – Weight gain, kg | |
| Albendazole 4.73 (1.49) | |
| Placebo 4.73 (1.52) P = 0.984 | |
| – Height gain, cm | |
| Albendazole 10.4 (2.47) | |
| Placebo 10.33 (2.06) P = 0.436 | |
| – MUAC gain, mm | |
| Albendazole 12.53 (7.61) | |
| Placebo 11.69 (7.87) P = 0.005 | |
| – Triceps gain, mm | |
| Albendazole 1.05 (1.05) | |
| Placebo 1.22 (1.08) P < 0.001 | |
| Notes | |
| – 93% had Ascaris or Trichuris and 61% had both. Mean egg counts were 7533 eggs g−1 Ascaris, 518 eggs g−1 Trichuris and 7 eggs g−1 hookworm | |
| – Treatment was effective against Ascaris and Trichuris, but became less effective over the course of the study. The first treatment cured 88.6% of Ascaris and 15.8% of Trichuris | |
| – At baseline 35% of boys and 24% of girls were stunted; 36% of boys and 39% of girls were underweight | |
| – At baseline control children were slightly heavier and had larger MUACs than treatment children | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Persson et al. (2001 )
Increase in serum beta‐carotene following dark green leafy vegetable supplementation in Mebendazole‐treated school children in Bangladesh. European Journal of Clinical Nutrition 55, 1–9.
| Design | Randomized trial. |
| Deworming alone or with carotene rich foods in vitamin A status. No group with no treatments. Infected children only + 21 with unhygienic toilets. Random assignment to groups, after stratification for anaemia | |
| Follow‐up | 57 days |
| Location | Panchargar and Thakurgaon, Bangladesh; rural |
| Age range | Mean ∼9.2 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 65% DGLV; 86% pumpkin; 68% mebendazole only |
| Trichuris trichiura: Not reported | |
| Hookworm: 32% DGLV; 25% pumpkin; 16% mebendazole only | |
| Treatments | Mebendazole only |
| vs. Mebendazole + DGLV | |
| vs. Mebendazole + pumpkin | |
| Mebendazole as 500 mg single dose 2 weeks before trial (day 1) | |
| Given 1 meal day−1, 6 days per week for 6 weeks | |
| Sample size | DGLV 37 |
| Pumpkin 36 | |
| Mebendazole only 37 | |
| Outcomes | Serum retinol increase |
| Serum beta‐carotene increase | |
| Findings | |
| Serum retinol change, µmol L−1 | |
| Mebendazole only −0.017 (95% CI −0.07, 0.035) | |
| Mebendazole + DGLV 0.066 (95% CI 0.002, 0.13) | |
| Mebendazole + pumpkin 0.030 (95% CI −0.035, 0.094) | |
| – Change from baseline significant in DGLV group only P = 0.04 | |
| – Mean change in serum retinol not significantly different between groups –anova P = 0.17 | |
| Serum β‐carotene change, µmol L−1 | |
| Mebendazole + DGLV 0.44 (95% CI 0.32, 0.55) | |
| Mebendazole + pumpkin 0.32 (95% CI 0.22, 0.42) | |
| Mebendazole only 0.20 (95% CI 0.14, 0.26) | |
| – All significantly increased from baseline; DGLV increase significantly greater than mebendazole alone P = 0.002 | |
| Notes | |
| – Mean egg count in children with Ascaris was 1800–3800 eggs g−1; cure rate was nearly complete | |
| – Sample size was too small (Discussion, page 7) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No untreated controls | |
Rousham & Mascie‐Taylor (1994 )
An 18‐month study of the effect of periodic anthelminthic treatment on the growth and nutritional status of pre‐school children in Bangladesh. Annals of Human Biology 21, 315–324.
| Design | Randomized controlled trial. |
| Randomization by village (13 villages – all in the area). All children included | |
| Follow‐up | 18 months |
| Location | Jamalpur, Bangladesh |
| Age range | 2–6 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 71% |
| Trichuris trichiura: 44% | |
| Hookworm: 10% | |
| Treatments | Mebendazole 500 mg single dose every 2 months |
| vs. Placebo | |
| Sample size | 688 mebendazole and 714 placebo children completed the trial, but anthropometry results presented for 1325 children; does not say how many in each group, Table 3 |
| Outcomes | MUAC change Z‐score of height‐for‐age change |
| Z‐score of weight‐for‐age change | |
| Z‐score of weight‐for‐height change | |
| Findings | |
| – MUAC change, cm | |
| Mebendazole 0.33 | |
| Placebo 0.23 NS | |
| – Z‐score of height‐for‐age change | |
| Mebendazole 0.25 | |
| Placebo 0.17 NS | |
| – Z‐score of weight‐for‐age change | |
| Mebendazole 0.03 | |
| Placebo 0.12 P < 0.05 | |
| – Z‐score of weight‐for‐height change | |
| Mebendazole −0.25 | |
| Placebo −0.05 P < 0.001 | |
| – In multiple regression analyses treatment group did not have a significant effect on anthropometry at any time point | |
| Notes | |
| – Significantly more mebendazole‐treated children had Trichuris than placebo children (65% vs. 23%) | |
| – Mean egg count in infected children was 813–1230 eggs g−1 Ascaris, 68–138 eggs g−1 Trichuris, and 58–82 eggs g−1 hookworm | |
| – All infections were light (<5000 eggs g−1 Ascaris, <1000 eggs g−1 Trichuris, <5000 eggs g−1 hookworm) | |
| – Mean Trichuris egg count was not significantly different between groups | |
| – Treatment led to significant decreases in all three worms (Table 2) | |
| – At baseline over 73% had z‐score of weight‐for‐age <−2 | |
| – Results also given at 6 and 12 months (Table 3) | |
| – No standard deviations given in Table 3 | |
| – Not used in the Cochrane meta‐analysis | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – no standard deviations given; not clear how clusters randomized | |
Effect of deworming on nutritional status of Ascaris infested slum children of Dhaka, Bangladesh. Indian Pediatrics 39, 1021–1026.
| Design | Randomized controlled trial. |
| Double blind. Ascaris‐infected children only | |
| Follow‐up | 16 weeks |
| Location | Dhaka, Bangladesh; slum |
| Age range | 2–12 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 78.5% |
| Trichuris trichiura: Not reported | |
| Hookworm: Not reported | |
| Treatments | Pyrantel 11 mg kg−1 single dose |
| vs. Placebo | |
| Sample size | Pyrantel 40 |
| Placebo 41 | |
| Outcomes | Weight change Weight‐for‐age % of median gain |
| Height change Height‐for‐age % of median gain | |
| Weight‐for‐height % of median gain | |
| Findings | |
| – Mean weight gain, treated 0.92 SD 0.84 kg | |
| Mean weight gain, placebo 0.54 SD 0.45 kg P < 0.05 | |
| – Mean height gain, treated 1.2 SD 1.5 cm | |
| Mean height gain, placebo 1.1 SD 0.7 cm P < 0.750 | |
| – Mean weight‐for‐age gain, treated 0.8 SD 4.6 % median | |
| Mean gain weight‐for‐age, placebo −0.1 SD 2.9 % median P < 0.05 | |
| – Mean height‐for‐age gain, treated −0.9 SD 1.1 % median | |
| Mean height‐for‐age gain, placebo −1.0 SD 0.4 % median P < 0.671 | |
| – Mean weight‐for‐height gain, treated 3.9 SD 5.6 % median | |
| Mean weight‐for‐height gain, placebo 2.0 SD 3.1 % median P < 0.085 | |
| Notes | |
| – Mean weight‐for‐age gain in placebos should be −1.0 or −0.9? (Table II) | |
| – Did not assess infection intensity or worm infections other than A. lumbricoides | |
| – At baseline mean weight‐for‐age ∼77% median, weight‐for‐height ∼90% median and height‐for‐age ∼92% median | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Treatment of Trichuris trichiura infections improves growth, spelling scores and school attendance in some children. Journal of Nutrition 125, 1875–1883.
| Design | Randomized controlled trial. |
| 14 schools. Children with Trichuris infection >1200 eggs g−1 recruited. Children randomly assigned to treatment or placebo. Double blind | |
| Follow‐up | 26 weeks |
| Location | Jamaica; rural and Kingston |
| Age range | 6–12 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 42% treatment; 50% placebo |
| Trichuris trichiura: 100% | |
| Hookworm: | |
| Treatments | Albendazole 400 mg on each of 2 consecutive days |
| vs. Placebo | |
| Treated at baseline, 12 and 24 weeks | |
| Sample size | Albendazole 206 |
| Placebo 201 | |
| Outcomes | Z‐score of height‐for‐age BMI |
| Findings | |
| – Z‐score of height‐for‐age | |
| Albendazole −0.48 SD 0.95 to −0.48 SD 0.97 | |
| Placebo −0.39 SD 0.90 to −0.41 SD 0.89 | |
| – No significant differences between the groups at either time point. P = 0.35 at baseline, P = 0.46 follow‐up | |
| – Body mass index (BMI), kg m−2 | |
| Albendazole 15.3 SD 1.3 to 15.6 SD 1.3 | |
| Placebo 15.5 SD 1.3 to 15.8 SD 1.4 | |
| – No significant differences between the groups at either time point. P = 0.07 at baseline, P = 0.25 follow‐up | |
| – There was a significant treatment‐by‐intensity interaction for BMI P = 0.009; children with low‐intensity Trichuris improved their BMI with treatment compared with placebo, but no difference for heavy infection vs. placebo (page 1880). No treatment effects seen with z‐score of height‐for‐age | |
| Notes | |
| – At baseline 46% had Ascaris: 10% light infections (<5000 eggs g−1), 27% moderate (5‐35 000 eggs g−1) and 9% heavy (>35 000 eggs g−1) | |
| – After follow‐up 50% of treated children were Trichuris free and 5% of them had infections >1200 eggs g−1. 7% of treated children still had Ascaris | |
| – Note baseline stunting (z‐score of height‐for‐age) values are not very low | |
| – Only paper to give data on BMI, so not used | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Relationships between Ascaris infection and growth of malnourished preschool children in Kenya. American Journal of Clinical Nutrition 33, 1165–1172.
| Design | Mothers in 2 villages invited to participate (62% did so; so no random selection). Children measured at visits I, II and III. All were treated at visits II and III. Those who had no worms throughout became controls; those who were worm positive at visits I and II but worm free at visit III became infected group |
| Follow‐up | 14 weeks |
| Location | Machakos district, Kenya |
| Age range | 12–72 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 100% treatment; 0% control |
| Trichuris trichiura: 2% treatment; 1% control | |
| Hookworm: 8% treatment; 7% control | |
| Treatments | Levamisole single dose; 40 mg <60 months, 80 mg >60 months |
| Sample size | Infected 61 |
| Control 125 (124 for triceps) | |
| Outcomes | Weight gain Weight‐for‐age % median gain |
| Triceps gain | |
| Findings | |
| – Mean weight gain in infected children 0.7 SD 0.4 kg | |
| Mean weight gain in control children 0.5 SD 0.5 kg P < 0.05 | |
| – Mean triceps gain in infected children 2.0 SD 0.9 mm | |
| Mean triceps gain in control children −1.1 SD 1.2 mm P < 0.0005 | |
| – Mean weight‐for‐age gain in infected children 1.6 SD 2.7% | |
| Mean weight‐for‐age gain in control children 0.7 SD 3.1% P < 0.10 | |
| – States that changes in MUAC were small (≤2 mm) so not reported | |
| Notes | |
| – Considered unethical to have infected but untreated children | |
| – Time between visits I and II is a baseline period; intervention starts at visit II | |
| – At baseline weight‐for‐age was ∼79.5%, height‐for‐age was ∼92% and weight‐for‐height ∼91% | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no untreated control group | |
Relationships of Schistosoma haematobium, hookworm and malarial infections and metrifonate treatment to growth of Kenyan school children. American Journal of Tropical Medicine and Hygiene 34, 1109–1118.
| Design | Randomized controlled trial. |
| Children with light/moderate (1–500 eggs per 10 mL adjusted) S. haematobium infections | |
| Follow‐up | 6 months |
| Location | Kwale district, Kenya |
| Age range | 6–16 y |
| Infection prevalence at baseline | Ascaris lumbricoides: Not reported |
| Trichuris trichiura: Not reported | |
| Hookworm: 93.9% | |
| Schistosoma haematobium: 100% | |
| Treatments | Metrifonate 7.5 mg kg−1 three times, 1–2 weeks apart |
| vs. Placebo | |
| Sample size | Metrifonate 201 |
| Placebo 198 | |
| Outcomes | Weight gain Weight‐for‐age % median gain |
| Height gain Height‐for‐age % median gain | |
| MUAC gain | |
| Triceps gain | |
| Findings | |
| – Mean weight gain in treated children 2.4 SE 0.08 kg | |
| Mean weight gain in control children 1.6 SE 0.09 kg P < 0.001 | |
| – Mean height gain in treated children 2.9 SE 0.07 cm | |
| Mean height gain in control children 2.8 SE 0.07 cm P < 0.079 | |
| – Mean MUAC gain in treated children 0.5 SE 0.04 cm | |
| Mean MUAC gain in control children 0.1 SE 0.04 cm P < 0.001 | |
| – Mean triceps gain in treated children 0.9 SE 0.06 mm | |
| Mean triceps gain in control children 0.0 SE 0.07 mm P < 0.001 | |
| – Mean weight‐for‐age gain in treated children 1.8 SE 0.19% | |
| Mean weight‐for‐age gain in control children −0.5 SE 0.22% P <0.001 | |
| – Mean height‐for‐age gain in treated children 0.1 SE 0.05% | |
| Mean height‐for‐age gain in control children 0.0 SE 0.09% P < 0.067 | |
| – Improvements seen in all treated children, not just those with highest egg counts (page 1112) | |
| Notes | |
| – Baseline nutritional status: % weight‐for‐age ∼80, % height‐for‐age ∼95.5 | |
| – Treatment produced a 94% egg reduction for S. haematobium and 67% egg reduction for hookworm | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Metrifonate used to treat Schistosoma spp., also treats hookworm | |
Single dose metrifonate or praziquantel treatment in Kenyan children. I. Effects on S. haematobium, hookworm, hemoglobin levels, splenomegaly, and hepatomegaly. American Journal of Tropical Medicine and Hygiene 41, 436–444.
And II.
Effects on growth in relation to S. haematobium and hookworm egg counts. American Journal of Tropical Medicine and Hygiene 41, 445–453.
| Design | Randomized controlled trial. |
| Children with blood in urine to compare metrifonate and praziquantel. Children individually randomized to placebo, met or praziquantel | |
| Follow‐up | 8 months |
| Location | Kwale district, Kenya |
| Age range | 6–17 y |
| Infection prevalence at baseline | Ascaris lumbricoides: Not reported |
| Trichuris trichiura: Not reported | |
| Hookworm: 94% metrifonate; 95% praziquantel; 89% placebo | |
| Schistosoma haematobium: 100% all groups | |
| Treatments | Metrifonate 10 mg kg−1 single dose |
| vs. Praziquantel 40 mg kg−1 single dose | |
| vs. Placebo | |
| Sample size | Metrifonate 103 |
| Praziquantel 105 | |
| Placebo 104 | |
| Outcomes | Haemoglobin change |
| Weight change % weight‐for‐age change | |
| Height change % height‐for‐age change | |
| MUAC change % weight‐for‐height change | |
| Triceps change | |
| Findings | |
| – Haemoglobin, g dL−1 | |
| Mean gain in metrifonate children 0.14 SE 0.089 | |
| Mean gain in praziquantel children −0.05 SE 0.086 | |
| Mean gain in placebo children −0.16 SE 0.088 | |
| – Weight, kg | |
| Mean gain in metrifonate children 3.3 SD 0.12 | |
| Mean gain in praziquantel children 3.1 SD 0.10 | |
| Mean gain in placebo children 1.9 SD 0.10 | |
| – Height, cm | |
| Mean gain in metrifonate children 3.6 SD 0.13 | |
| Mean gain in praziquantel children 3.5 SD 0.11 | |
| Mean gain in placebo children 3.4 SD 0.11 | |
| – MUAC, cm | |
| Mean gain in metrifonate children 0.9 SD 0.05 | |
| Mean gain in praziquantel children 0.9 SD 0.05 | |
| Mean gain in placebo children 0.2 SD 0.04 | |
| – Triceps, mm | |
| Mean gain in metrifonate children 1.5 SD 0.11 | |
| Mean gain in praziquantel children 1.4 SD 0.09 | |
| Mean gain in placebo children 0.0 SD 0.08 | |
| – % weight‐for‐age | |
| Mean gain in metrifonate children 2.4 SD 0.27 | |
| Mean gain in praziquantel children 2.0 SD 0.24 | |
| Mean gain in placebo children −1.3 SD 0.25 | |
| – % height‐for‐age | |
| Mean gain in metrifonate children 0.1 SD 0.09 | |
| Mean gain in praziquantel children −0.2 SD 0.09 | |
| Mean gain in placebo children −0.3 SD 0.08 | |
| – % weight‐for‐height | |
| Mean gain in metrifonate children 3.4 SD 0.32 | |
| Mean gain in praziquantel children 3.5 SD 0.33 | |
| Mean gain in placebo children −0.2 SD 0.27 | |
| – All significantly greater than placebo except height in both and height‐for‐age in praziquantel, P = 0.0001 (page 448) | |
| – But two drugs not significantly different from each other (page 447) | |
| Notes | |
| – Geometric mean hookworm egg count 1029–1326 eggs g−1; geometric mean count S. haematobium 38–57 eggs | |
| – Metrifonate produced a 76% egg reduction for S. haematobium and 59% egg reduction for hookworm; praziquantel produced a 99% egg reduction for S. haematobium and 23% egg reduction for hookworm (Table 1); hookworm egg count fall only significant for met group; neither treatment significantly reduced hookworm prevalence but both significantly reduced S. haematobium prevalence | |
| – Those with heavy S. haematobium infection excluded (>500 eggs per 10 mL urine); also severe anaemics (haemoglobin <8 g dL−1) | |
| – At baseline mean haemoglobin was ∼11.4 g dL−1, mean weight‐for‐age ∼73%, mean height‐for‐age ∼93% and mean weight‐for‐height ∼90% | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – praziquantel given; metrifonate used which treats Schistosoma spp. as well as hookworm; no specific treatment for intestinal worms only | |
Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichuria and Ascaris lumbricoides infections. American Journal of Tropical Medicine and Hygiene 41, 78–87.
| Design | Randomized controlled trial. |
| One school; all children included. Randomly allocated within sex to treatment or placebo | |
| Follow‐up | 6 months |
| Location | Kwale district, Kenya |
| Age range | 6–16 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 49% |
| Trichuris trichiura: 97% | |
| Hookworm: 87% | |
| Treatments | Albendazole 400 mg single dose |
| vs. Placebo | |
| Sample size | Albendazole 78 |
| Placebo 72 | |
| Outcomes | Weight change % weight‐for‐age change |
| Height change % height‐for‐age change | |
| MUAC change % weight‐for‐height change | |
| Triceps change | |
| Findings | |
| – Weight change, kg | |
| Albendazole 2.1 SE 0.09 | |
| Placebo 0.8 SE 0.10 P = 0.0002 | |
| – Height change, cm | |
| Albendazole 2.8 SE 0.09 | |
| Placebo 2.2 SE 0.10 P = 0.0002 | |
| – MUAC change, cm | |
| Albendazole 0.7 SE 0.05 | |
| Placebo 0.2 SE 0.06 P = 0.0002 | |
| – Triceps change, mm | |
| Albendazole 1.0 SE 0.08 | |
| Placebo 0.2 SE 0.08 P = 0.0002 | |
| – % weight‐for‐age change | |
| Albendazole 1.8 SE 0.29 | |
| Placebo 2.7 SE 0.34 P = 0.0002 | |
| – % height‐for‐age change | |
| Albendazole −0.2 SE 0.06 | |
| Placebo −0.7 SE 0.08 P = 0.0002 | |
| – % weight‐for‐height change | |
| Albendazole 3.3 SD 0.41 | |
| Placebo −1.0 SD 0.44 P = 0.0002 | |
| – Effects seen in all children, – not only those with heavy infections | |
| – Found linear relationships between increase in growth rate and decrease in intensity of infection (page 82) | |
| Notes | |
| – At follow‐up 13% of treated children had Ascaris, 91% had Trichuris and 77% had hookworm. % egg reduction in treated children was 91% for Ascaris, 28% for Trichuris and 67% for hookworm (Table 1) | |
| – At baseline mean % weight‐for‐age ∼73%, height‐for‐age ∼92%, weight‐for‐height ∼90% | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Weight gain of Kenyan school children infected with hookworm, Trichuris trichuria and Ascaris lumbricoides is improved following once‐ or twice‐yearly treatment with albendazole. Journal of Nutrition 123, 656–665.
| Design | Randomized controlled trial. |
| One school. All children included. Random allocation by descending egg count to placebo, one or two treatments | |
| Follow‐up | 8.2 months |
| Location | Kwale district, Kenya |
| Age range | 6–15 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 35% one treatment; 26% two treatments; 32% placebo |
| Trichuris trichiura: 90% one treatment; 81% two treatments; 92% placebo | |
| Hookworm: 85% one treatment; 86% two treatments; 88% placebo | |
| Treatments | Albendazole 600 mg then placebo |
| vs. Albendazole 600 mg then albendazole 600 mg | |
| vs. Placebos | |
| Treatment as single dose, repeated at 3.6 months | |
| Sample size | Albendazole once 96 |
| Albendazole twice 95 | |
| Placebo 93 | |
| Outcomes | Weight change % weight‐for‐age change |
| Height change % height‐for‐age change | |
| MUAC change % weight‐for‐height change | |
| Triceps change | |
| Findings | |
| – Weight change, kg | |
| Albendazole once 3.3 SE 0.18 P = 0.0001 | |
| Albendazole twice 3.1 SE 0.14 P = 0.0001 | |
| Placebo 2.2 SE 0.12 | |
| – Height change, cm | |
| Albendazole once 3.8 SE 0.12 NS | |
| Albendazole twice 3.6 SE 0.11 NS | |
| Placebo 3.7 SE 0.12 | |
| – MUAC change, cm | |
| Albendazole once 0.8 SE 0.05 P = 0.0001 | |
| Albendazole twice 0.7 SE 0.05 P = 0.0001 | |
| Placebo 0.3 SE 0.04 | |
| – Triceps change, mm | |
| Albendazole once 2.0 SE 0.11 P = 0.0001 | |
| Albendazole twice 2.0 SE 0.12 P = 0.0001 | |
| Placebo 0.2 SE 0.08 | |
| – % weight‐for‐age change | |
| Albendazole once 1.9 SE 0.36 P = 0.0001 | |
| Albendazole twice 1.3 SE 0.30 P = 0.0001 | |
| Placebo −1.4 SE 0.28 | |
| – % height‐for‐age change | |
| Albendazole once −0.2 SE 0.08 NS | |
| Albendazole twice −0.3 SE 0.08 NS | |
| Placebo −0.4 SE 0.07 | |
| – % weight‐for‐height change | |
| Albendazole once 2.8 SE 0.36 P = 0.0001 | |
| Albendazole twice 2.6 SE 0.35 P = 0.0001 | |
| Placebo −0.3 SE 0.30 | |
| – The two treatment groups did not differ from each other (page 661) | |
| – Growth effects were general, affecting all children | |
| Notes | |
| – Placebo group had a significantly higher initial Trichuris intensity than the twice treated group | |
| – At follow‐up 16% and 5% of treated children had Ascaris, 81% and 66% had Trichuris and 45% and 23% had hookworm (all once and twice treated respectively) (Table 1). Significant egg reductions for all treated children and all worms (Table 2) | |
| – At baseline mean % weight‐for‐age ∼81%, height‐for‐age ∼94.5%, weight‐for‐height ∼94% | |
| – Also results at 3.6 months for parasitology | |
| – Stephenson et al. (1993b) is a substudy of this one | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichuria and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. Journal of Nutrition 123, 1036–1046.
| Design | Randomized controlled trial. |
| One school. Boys selected who had adequate haemoglobin level (>80 g L−1) and had a parasite infection, but vs. high hookworm (>20 000 eggs g−1) excluded. Randomly allocated by descending hookworm egg count to treatment or placebo. | |
| Follow‐up | 4 months |
| Location | Kwale district, Kenya |
| Age range | 7–13 y; boys only |
| Infection prevalence at baseline | Ascaris lumbricoides: 41% |
| Trichuris trichiura: 98% | |
| Hookworm: 96% | |
| Treatments | Albendazole 600 mg single dose |
| vs. Placebo | |
| Sample size | Albendazole 27 |
| Placebo 26 | |
| Outcomes | Weight change % weight‐for‐age change |
| Height change % height‐for‐age change | |
| MUAC change % weight‐for‐height change | |
| Triceps change | |
| Haemoglobin change | |
| Findings | |
| – Weight change, kg | |
| Albendazole 1.6 SE 0.15 P = 0.0002 | |
| Placebo 0.6 SE 0.08 | |
| – Height change, cm | |
| Albendazole 2.0 SE 0.19 P = 0.003 | |
| Placebo 1.4 SE 0.08 | |
| – MUAC change, cm | |
| Albendazole 0.3 SE 0.06 P = 0.0002 | |
| Placebo 0.0 SE 0.05 | |
| – Triceps change, mm | |
| Albendazole 1.0 SE 0.09 P = 0.0002 | |
| Placebo 0.0 SE 0.10 | |
| – % weight‐for‐age change | |
| Albendazole 1.0 SE 0.42 P = 0.0002 | |
| Placebo −2.0 SE 0.24 | |
| – % height‐for‐age change | |
| Albendazole −0.1 SE 0.13 P = 0.0015 | |
| Placebo −0.6 SE 0.06 | |
| – % weight‐for‐height change | |
| Albendazole 1.6 SD 0.49 P = 0.0002 | |
| Placebo −0.6 SD 0.28 | |
| – Haemoglobin change, g L−1 | |
| Albendazole −6 SE 1.0 P = 0.002 | |
| Placebo −2 SE 1.2 | |
| Notes | |
| – At baseline ∼40% had Ascaris, ∼98% had Trichuris and 96% had hookworm | |
| – At follow‐up 18% of treated children had Ascaris, 85% had Trichuris and 44% had hookworm (Table 1). Significant egg reductions for treated children and all worms (Table 1) | |
| – At baseline mean % weight‐for‐age ∼80%, height‐for‐age ∼95%, weight‐for‐height ∼93% | |
| – Authors do not think the haemoglobin change is biologically significant | |
| – This is a substudy of Stephenson et al. (1993a); entered in the Cochrane meta‐analysis separately for the anthropometry results. They used the haemoglobin though | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
School‐based deworming program yields small improvement in growth of Zanzibari school children after one year. Journal of Nutrition 127, 2187–2193.
| Design | Randomized controlled trial. |
| 12/72 schools randomly selected then schools randomly allocated to three groups (Fig. 1). All children included | |
| Follow‐up | 1 y |
| Location | Zanzibar, Tanzania |
| Age range | School aged, average 10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 66.5% twice yearly treatment; 76.4% thrice yearly treatment; 73.0% control |
| Trichuris trichiura: 96.7% twice yearly treatment; 97.3% thrice yearly treatment; 94.7% control | |
| Hookworm: 94.5% twice yearly treatment; 95.8% thrice yearly treatment; 91.2% control | |
| Schistosoma haematobium: 21.3% twice yearly treatment; 39.5% thrice yearly treatment; 31.9% control | |
| Treatments | Mebendazole 500 mg single dose twice a year |
| vs. Mebendazole 500 mg single dose three times a year | |
| vs. No treatment | |
| Sample size | Mebendazole‐2 990 |
| Mebendazole‐3 1019 | |
| No treatment 1054 | |
| Outcomes | Weight gain |
| Height gain | |
| Findings | |
| – Weight gain, kg | |
| <10 y ≥10 y | |
| Control 2.11 SE 0.08 3.01 SE 0.11 | |
| Mebendazole 2 times 2.38 SE 0.08 3.20 SE 0.12 | |
| Mebendazole 3 times 2.31 SE 0.08 3.04 SE 0.12 | |
| – Mebendazole 2 times vs. placebo in <10 y: P < 0.05 | |
| – Height gain, cm | |
| <10 y ≥10 y | |
| Control 4.29 SD 0.07 4.74 SD 0.08 | |
| Mebendazole 2 times 4.42 SD 0.07 4.67 SD 0.08 | |
| Mebendazole 3 times 4.59 SD 0.07 4.81 SD 0.08 | |
| – Mebendazole 3 times vs. placebo in <10 y: P < 0.01 | |
| – In under 10 y, children with higher baseline z‐score of height‐for‐age benefited more from deworming, P < 0.005 for both groups vs. controls (page 2190 and Fig. 2). Stunted children wormed three times did show a significant height gain though | |
| – Baseline prevalence and intensity of infection did not predict growth responses | |
| Notes | |
| – Geometric mean eggs g−1 were 332–601 for hookworm, 531–625 for Trichuris and 149–335 for Ascaris for the three groups | |
| – The treatment groups had significantly higher prevalence of hookworm and Trichuris than controls (Table 1) | |
| – At baseline ∼30% of under 10s were stunted and ∼22% had BMI <5th centile; among over 10s, ∼64% were stunted and ∼48% had low BMI (Table 1). Prevalence of anaemia was 62.3% | |
| – Treatment was effective against Ascaris, but less so against Trichuris and hookworm. Both treatment regimes significantly reduced prevalence and intensity of the three infections. Dose response effect seen (Table 2) | |
| – Authors comment that the gains achieved are relatively small and do not bring the children's growth into line with international standards. They also note that the greater growth seen in the least stunted children suggests other factors must be slowing growth in stunted children | |
| – They also discuss the possibility that worming causes a spurt of weight gain that is not sustained beyond 6 months and that the treatments’ limited effect on Trichuris may be having an effect | |
| – The haemoglobin results are reported in Stoltzfus et al. (1998) | |
| – The paper uses ‘placebo’, but in Stoltzfus et al. (1998) (same trial) they state that placebos were not given in non‐programme schools | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Effects of the Zanzibar school‐based deworming program on iron status of children. American Journal of Clinical Nutrition 127, 179–186.
| Design | Randomized controlled trial. |
| 12/72 schools randomly selected then schools randomly allocated to three groups. All children included | |
| Follow‐up | 1 y |
| Location | Zanzibar, Tanzania |
| Age range | School aged, median 10 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 66.7% twice yearly treatment; 75.8% thrice yearly treatment; 72.7% control |
| Trichuris trichiura: 96.5% twice yearly treatment; 97.1% thrice yearly treatment; 94.8% control | |
| Hookworm: 94.4% twice yearly treatment; 95.7% thrice yearly treatment; 91.0% control | |
| Treatments | Mebendazole 500 mg single dose twice a year |
| vs. Mebendazole 500 mg single dose three times a year | |
| vs. No treatment | |
| Sample size | Mebendazole‐2 952 |
| Mebendazole‐3 970 | |
| Controls 1002 | |
| Outcomes | Haemoglobin change |
| Findings | |
| – Haemoglobin change, g L−1 | |
| Control 11.3 (SE 1.7) | |
| Mebendazole‐2 10.3 (SE 1.7) | |
| Mebendazole‐3 12.7 (SE 1.7) | |
| – All groups improved significantly from baseline; no significant increase of treatment groups over controls | |
| – In children with hookworm infections ≥2000 eggs g−1 both treatment regimes had a significant impact on moderate to severe anaemia incidence (Table 3) | |
| Notes | |
| – Mean eggs g−1 were 321–583 for hookworm, 527–614 for Trichuris and 152–314 for Ascaris for the three groups. 20–35% had microhaematuria | |
| – Groups had significantly different prevalences and intensities of infection at baseline (Table 1) | |
| – At baseline 48.5% were stunted and ∼60% were anaemic (haemoglobin <110 g L−1); mean haemoglobin was 104–107 g L−1. ∼57% had malaria parasitaemia | |
| – Treatment was effective against Ascaris, but less so against Trichuris and hookworm (pages 181–2) | |
| – Severe anaemics (haemoglobin <70 g L−1) were not included | |
| – Also has results for low haemoglobin prevalence, protoporphyrin and ferritin (Table 2) | |
| – Authors suggest that an unusually good harvest may explain the lack of effect. They also raise the point of needing to supply additional iron to do more than halt or slow a decline in iron status | |
| This is the same study as Stoltzfus et al. (1997) | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. Journal of Nutrition 134, 348–356.
| Design | Randomized controlled trial. |
| Iron and/or mebendazole; randomization by blocks of households to iron or placebo, then randomized within these two groups to deworming or placebo (Fig. 1) | |
| Follow‐up | 12 months |
| Location | Zanzibar, Tanzania |
| Age range | 6–71 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 28% mebendazole and 35% placebo, children <30 months; 50% mebendazole and 52% placebo, children ≥30 months |
| Trichuris trichiura: 45% mebendazole and 50% placebo, children <30 months; 86% mebendazole and 87% placebo, children ≥30 months | |
| Hookworm: 26% mebendazole and 35% placebo, children <30 months; 71% mebendazole and 68% placebo, children ≥30 months | |
| Treatments | Iron daily for 1 y and placebo |
| vs. Iron 10 mg day−1 and mebendazole 500 mg every 3 months | |
| vs. Mebendazole 500 mg and placebo | |
| vs. Placebos | |
| Sample size | Iron + mebendazole 114 |
| Iron 118 | |
| Mebendazole 106 | |
| Placebos 121 | |
| Mebendazole 220 | |
| Iron 232 | |
| No mebendazole 239 | |
| No iron 227 | |
| Outcomes | Haemoglobin |
| Findings | |
| – Mean haemoglobin at baseline mebendazole (n220) 91 SD 11 g L−1 | |
| Mean haemoglobin at 12 months mebendazole 100 SD 16 g L−1 | |
| – Mean haemoglobin at baseline no mebendazole (n239) 91 SD 12 g L−1 | |
| Mean haemoglobin at 12 months no mebendazole 99 SD 16 g L−1 | |
| – No significant treatment effects on haemoglobin or on anaemia, although prevalence of moderate anaemia about 30% lower after 12 months than in children without treatment (Table %) | |
| Notes | |
| – At baseline 38% stunted, 31.2% underweight and 3.8% wasted; 94% had mild anaemia | |
| – Mebendazole highly effective in controlling Ascaris, less effective against Trichuris and least effective against hookworm (Table 3) | |
| Severe anaemics excluded (<70 g L−1) | |
| Has some anthropometry results but given as %s with MUAC <5th centile, %s with z‐score of weight‐for‐height <−1 and %s with z‐score of height‐for‐age <−2 and as adjusted odds ratios (Table 4); in children <30 months these are quite substantial and significant for MUAC and z‐score of weight‐for‐height | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Periodic deworming with albendazole and its impact on growth status and diarrhoeal incidence among children in an urban slum of India. Transactions of the Royal Society of Tropical Medicine and Hygiene 99, 261–267.
| Design | Randomized controlled trial, double blind |
| Follow‐up | 9 months |
| Location | Kolkata, India; slum |
| Age range | 2–5 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 53.9% albendazole; 51.8% control |
| Trichuris trichiura: Not reported | |
| Hookworm: Not reported | |
| Treatments | Albendazole 400 mg; two single doses 6 months apart |
| vs. Placebo | |
| Sample size | Albendazole 342 |
| Placebo 340 | |
| Outcomes | Proportional weight gain |
| Findings | |
| – Albendazole group: 57% gained <10% and 43% gained >10% | |
| Placebo group: 70.5% gained <10% and 29.5% gained >10% | |
| – P = 0.02 comparing >10% weight gain between the 2 groups | |
| – Actual weight gain is only given as a graph (Fig. 1), but difference between groups is significant at 3, 6 and 9 months | |
| Note | |
| – At baseline Ascaris∼53%; albendazole reduced prevalence to 24%. Egg counts not given | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: results not presented in a usable way – weight gain as a graph | |
Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. Journal of Nutrition 126, 451–457.
| Design | Randomized controlled trial of deworming in addition to vitamin A supplements; double blind; children individually randomized; all Ascaris politive; excluded if also had hookworm |
| Follow‐up | 3–4 weeks after first modified relative dose response (MRDR) test |
| Location | Bogor, Indonesia; urban and rural |
| Age range | 0.6–6.6 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 100% all groups |
| Trichuris trichiura: 17–33% | |
| Hookworm: Not reported | |
| Treatments | 1 week before first MRDR Straight after first MRDR |
| A: – Albendazole | |
| B: – Albendazole + vitamin A | |
| C: Albendazole Vitamin A | |
| D: Albendazole – | |
| E: – Vitamin A | |
| F: – – | |
| Albendazole 400 mg single dose | |
| Vitamin A single dose of 210 µmol | |
| Sample size | A 52 D 51 |
| B 52 E 49 | |
| C 54 F 50 | |
| Outcomes | Serum retinol increase |
| Modified relative dose response test | |
| Findings | |
| – Serum retinol, µmol L−1 | |
| A: 0.71 SD 0.21 to 0.68 SD 0.21 | |
| B: 0.80 SD 0.26 to 0.82 SD 0.23 | |
| C: 0.70 SD 0.26 to 0.72 SD 0.19 | |
| D: 0.73 SD 0.25 to 0.71 SD 0.19 | |
| E: 0.74 SD 0.21 to 0.72 SD 0.19 | |
| F: 0.74 SD 0.20 to 0.73 SD 0.20 | |
| – Albendazole alone did not have a significant impact P = 0.724 (page 454) | |
| – Statistical interaction between treatment and supplementation P = 0.086 | |
| – No benefit of giving albendazole a week before the supplement vs. at the same time | |
| – Dehydroretinol to retinol ratio (DR/R) | |
| A: 0.065 SD 0.059 to 0.057 SD 0.054 | |
| B: 0.054 SD 0.038 to 0.029 SD 0.018 | |
| C: 0.056 SD 0.041 to 0.036 SD 0.018 | |
| D: 0.059 SD 0.045 to 0.057 SD 0.037 | |
| E: 0.060 SD 0.062 to 0.033 SD 0.014 | |
| F: 0.056 SD 0.032 to 0.050 SD 0.031 | |
| Albendazole alone did not have a significant impact P = 0.370, nor did the statistical interaction of treatment and supplementation P = 0.752 (page 454) | |
| Notes | |
| – Trichuris co‐infection ranged from 17% to 33%; 60% had light Ascaris infections (1–4999 eggs g−1) and 30% moderate infections (5000–49 999 eggs g−1) | |
| – Also has results for % children with serum retinol <0.35 and <0.70 (Table 4) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Tanumihardjo & Permaesih (2004 )
Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming treatments. European Journal of Clinical Nutrition 58, 1223–1230.
| Design | Nested within Tanumihardjo et al. (1996b) paper but has some additional children with Trichuris. All infected with Ascaris and/or Trichuris and all had received routine vitamin A in the month preceding this study. Randomized into 3 groups |
| Follow‐up | 3–4 weeks |
| Location | Bogor, Indonesia; urban and rural |
| Age range | Mean 3.4 SD 1.1 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 100% |
| Trichuris trichiura: 27.5% | |
| Hookworm: | |
| Treatments | A: Albendazole 400 mg 1 week before 1st modified relative dose response (MRDR) test |
| B: Albendazole 400 mg straight after 1st MRDR test | |
| C: Not dewormed | |
| No additional vitamin A supplements | |
| Sample size | A 21 |
| B 19 | |
| C 11 | |
| Outcomes | Haemoglobin |
| MRDR | |
| Findings | |
| – Haemoglobin, g L−1 | |
| A 110 SD 8.6 to 121 SD 12.7 Follow‐up 4 weeks since albendazole | |
| B 109 SD 9.7 to 112 SD 10.9 Follow‐up 3 weeks since albendazole | |
| C 114 SD 11.5 to 115 SD 9.6 | |
| – Significant increase in haemoglobin in group A | |
| – MRDR | |
| A: 0.023 SD 0.014 to 0.025 SD 0.013 Follow‐up 4 weeks since albendazole | |
| B: 0.019 SD 0.019 to 0.024 SD 0.024 Follow‐up 3 weeks since albendazole | |
| C: 0.021 SD 0.010 to 0.023 SD 0.015 | |
| – No significant differences in MRDR values | |
| Notes | |
| – Group 1 is observational; group 2 experimental – group 2 results here | |
| – Baseline serum retinol 0.81 µmol L−1, haemoglobin 110 g L−1; no child had vitamin A deficiency at baseline or follow‐up based on MRDR results | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Taylor et al. (2001 )
The effect of different anthelmintic treatment regimens combined with iron supplementation on the nutritional status of schoolchildren in KwaZulu‐Natal, South Africa: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 95, 211–216.
| Design | Randomized controlled trial. |
| Two doses of albendazole with/out iron, double blind; 6‐group factorial design. Albendazole always given with praziquantel. All children included | |
| Follow‐up | 12 months |
| Location | Kwa‐Zulu Natal, South Africa |
| Age range | 6–15 y (but no girls >12 y) |
| Infection prevalence at baseline | Ascaris lumbricoides: 55.9% |
| Trichuris trichiura: 83.6% | |
| Hookworm: 59.4% | |
| Schistosoma haematobium: 43.4% | |
| Treatments | 1: Albendazole 400 mg + praziquantel + iron |
| 2: Albendazole 400 mg + praziquantel + placebo | |
| 3: Albendazole 1200 mg + praziquantel + iron | |
| 4: Albendazole 1200 mg + praziquantel + placebo | |
| 5: Placebos + iron | |
| 6: Placebos | |
| Albendazole‐1200 was 400 mg given for 3 days | |
| Iron supplement: 65 mg ferrous iron + 100 µg folate; weekly for 10 weeks | |
| All treatments repeated at 6 months | |
| Sample size | 1: 34 4: 34 |
| 2: 41 5: 64 | |
| 3: 41 6: 61 (for haemoglobin) | |
| Outcomes | Haemoglobin change Z‐score of height‐for‐age |
| Z‐score of weight‐for‐age | |
| Findings | |
| At 12 months: haemoglobin change, | |
| g L−1 95% CI P | |
| 1 −0.9 −4.0, 2.1 0.54 | |
| 2 0.1 −3.7, 2.1 0.94 | |
| 3 3.5 0.5, 6.5 0.02 | |
| 4 −3.8 −6.9, −0.7 0.02 | |
| 5 −2.4 −4.7, −0.1 0.005 | |
| 6 −5.8 −8.1, −3.4 0.04 | |
| – At 6 months, groups 1–4 maintained their haemoglobin, while the other two groups showed significant declines (page 213), despite group 5 having iron | |
| – Note that at 12 months, only group 3 showed benefit; group 4 (same treatment but without iron) did not | |
| – anova showed a significant effect of albendazole‐1200 + iron at 12 months, P < 0.001 | |
| – Z‐score of height‐for‐age and z‐score of weight‐for‐age at 12 months were not associated with group or with helminth infections (page 213) | |
| Notes | |
| – At baseline mean z‐score of height‐for‐age −0.6 and z‐score of weight‐for‐age −0.62, anaemia 33.5% (<120 g L−1) | |
| – At baseline 44.4% had blood in urine; area endemic for schistosomiasis, hookworm and Trichuris | |
| – At 12 months Ascaris was 11% and hookworm 0.6% | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No – praziquantel given in addition to albendazole to all treated groups | |
A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 523–528.
| Design | Randomized controlled trial. 21/24 villages in study area randomly allocated to 8 intervention and 13 control. All children included. Not blind |
| Follow‐up | 2 y |
| Location | Yangon Division, Myanmar; rural |
| Age range | 2–12 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 80.8% intervention; 83.0% control |
| Trichuris trichiura: 5.2% intervention; 7.0% control | |
| Hookworm: 1.8% intervention; 1.0% control | |
| Strongyloides stercoralis: 1.5% intervention; 4.5% control | |
| Treatments | Levamisole according to manufacturer's instructions every 3 months |
| vs. No treatment | |
| Sample size | Levamisole 210 |
| No treatment 205 | |
| Outcomes | Weight gain |
| Height gain | |
| Findings | |
| – Weight gain, kg | |
| Levamisole 3.58 (SD 1.28) | |
| Controls 2.65 (SD 1.04) P = 0.001 | |
| – Height gain, cm | |
| Levamisole 11.31 (SD 2.04) | |
| Controls 10.66 (SD 1.77) P = 0.001 | |
| – Effects on height not significant until 12 months after baseline for 2–5‐y‐olds and until 18 months for 6–10‐y‐olds; effects on weight significant after 6 months | |
| Notes | |
| – At baseline ∼82% had Ascaris, ∼6% had Trichuris and ∼1% had hookworm | |
| – At baseline ∼57% stunted and ∼48% underweight | |
| – Also gives results broken down by age and interim time points (Tables 3,4)(Note that these tables use ‘height‐for‐age’ and ‘weight‐for‐age’ but presumably mean height and weight, not standardized measurements) | |
| – Not used in the Cochrane meta‐analysis; unclear why – possibly high loss to follow‐up (66%) | |
| – Additional data on most stunted children in Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 433 | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: Yes | |
Effect of removing Ascaris on the growth of Guatemalan schoolchildren. Pediatrics 97, 871–876.
| Design | Randomized controlled trial. Children from all (?) local schools stratified by sex and age then randomized to two groups. All children included |
| Follow‐up | 25 weeks |
| Location | Sacatepéquez, Guatemala; highlands |
| Age range | School aged, <12 y |
| Infection prevalence at baseline | Ascaris lumbricoides: 91% treatment; 92% control |
| Trichuris trichiura: 85% treatment; 78% control | |
| Hookworm: Not reported | |
| Treatments | Albendazole 400 mg single dose |
| vs. Placebo | |
| Treated at baseline and 12 weeks | |
| Sample size | Wt, z‐score of Ht, z‐score of Z‐score of MUAC |
| weight‐for‐age height‐for‐age weight‐for‐height | |
| Albendazole 116 116 70 106 | |
| Placebo 110 111 66 101 | |
| Outcomes | Weight change Z‐score of weight‐for‐age change |
| Height change Z‐score of height‐for‐age change | |
| MUAC change Z‐score of weight‐for‐height change | |
| Findings[means (SEM)] | |
| – Weight change, kg | |
| Albendazole 1.82 (SE 0.08) | |
| Placebo 1.69 (SE 0.07) P = 0.21 | |
| – Height change, cm | |
| Albendazole 2.45 (SE 0.07) | |
| Placebo 2.39 (SE 0.07) P = 0.55 | |
| – MUAC change, cm | |
| Albendazole 0.60 (SE 0.05) | |
| Placebo 0.52 (SE 0.05) P = 0.28 | |
| – Z‐score of weight‐for‐age change | |
| Albendazole 0.12 (SE 0.01) | |
| Placebo 0.09 (SE 0.01) P = 0.067 | |
| – Z‐score of height‐for‐age change | |
| Albendazole 0.00 (SE 0.01) | |
| Placebo −0.01 (SE 0.01) P = 0.30 | |
| – Z‐score of weight‐for‐height change | |
| Albendazole 0.25 (SE 0.04) | |
| Placebos 0.22 (SE 0.04) P = 0.59 | |
| Notes | |
| – At baseline 91% had Ascaris and 82% had Trichuris. No hookworm detected. Geometric mean eggs g−1 were 659–832 for Trichuris and 21 528–21 677 for Ascaris | |
| – At baseline mean z‐score of height‐for‐age was −2.68 and mean z‐score of weight‐for‐height was 0.48 | |
| – Treatment was effective against Ascaris, but less so against Trichuris. Ascaris prevalence fell from 91% to 17%, but reinfection was considerable (page 873, Table 1) | |
| – Also presents results at 12 weeks; none of the anthropometry changes were significant at 12 weeks either | |
| – The authors think their sample size was too small | |
| Included in Cochrane review of 2000: Yes | |
| Included in present meta‐analysis: Yes | |
Ascaris and growth rates: a randomized trial of treatment. American Journal of Public Health 69, 987–991.
| Design | Randomized controlled trial. Random allocation of all children attending a clinic to treatment or placebo |
| Follow‐up | 1 y |
| Location | Tanzania; village, mountainous area |
| Age range | 6–91 months |
| Infection prevalence at baseline | Ascaris lumbricoides: 53% |
| Trichuris trichiura: Not reported | |
| Hookworm: Not reported | |
| Treatments | Levamisole 2.5 mg kg−1 every 3 months |
| vs. Placebo | |
| Sample size | Weight gain data for 273 children and length gain for 268, but does not say how many in treatment and placebo groups (Table 3) |
| Outcomes | Weight gain |
| Length gain | |
| Findings | |
| – Levamisole gained 2.08 kg per year and placebo 1.92 kg per year P = 0.06 | |
| – Levamisole gained 7.58 cm per year and placebo 7.73 cm per year NS | |
| – No standard deviations given and unclear what number in each group | |
| Notes | |
| – At baseline 53% had stools positive for Ascaris; mean egg count was 6300 eggs g−1. 11% had hookworm | |
| – At baseline mean nutritional status was 0.79 (ratio of observed/expected weight‐for‐age) | |
| Included in Cochrane review of 2000: No | |
| Included in present meta‐analysis: No: no standard deviations given | |
Footnotes
The Japanese Organization for International Cooperation in Family Planning (JOICFP).
Downloaded from http://www.cc-ims.net/RevMan
References
- Adams E., Stephenson L., Latham M. & Kinoti S. (1994) Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. Journal of Nutrition 124, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Aguayo V.M. (2000) School‐administered weekly iron supplementation – effect on the growth and hemoglobin status of non‐anemic Bolivian school‐age children. A randomized placebo‐controlled trial. European Journal of Nutrition 39, 263–269. [DOI] [PubMed] [Google Scholar]
- Al‐Mekhlafi H.M.S., Shaik A., Ismail M.G., Sa’iah A., Azlin M., Aini U.N. et al (2005) Protein‐energy malnutrition and soil‐transmitted helminthiases among Orang Asli children in Selangor, Malaysia. Asia Pacific Journal of Clinical Nutrition 14, 188–194. [PubMed] [Google Scholar]
- Albonico M., Smith P.G., Hall A., Chwaya H.M., Alawi K.S. & Savioli L. (1994) A randomized controlled trial comparing mebendazole and albendazole against Ascaris, Trichuris and hookworm infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 585. [DOI] [PubMed] [Google Scholar]
- Albonico M., Stoltzfus R.J., Savioli L., Tielsch J.M., Chwaya H.M., Ercole E. et al (1998) Epidemiological evidence for a differential effect of hookworm species, Ancylostoma duodenale or Necator americanus, on iron status of children. International Journal of Epidemiology 27, 530–537. [DOI] [PubMed] [Google Scholar]
- Albonico M., Bickle Q., Haji H.J., Ramsan M., Khatib K.J., Montresor A. et al (2002) Evaluation of the efficacy of pyrantel‐oxantel for the treatment of soil‐transmitted nematode infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albonico M., Montresor A., Savioli L., Bickle Q., Ramsan M. & Taylor M. (2003) Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bulletin of the World Health Organization 81, 343–352. [PMC free article] [PubMed] [Google Scholar]
- Alderman H., Konde‐Lule J., Sebuliba I., Bundy D. & Hall A. (2006) Effect on weight gain of routinely giving albendazole to preschool children during ‘Child Health Days’ in Uganda: cluster randomised controlled trial. British Medical Journal 333, 122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M. & May R.M. (1991) Infectious Diseases of Humans. Dynamics and Control. Oxford University Press: Oxford. [Google Scholar]
- Arca M.J., Gates R.L., Groner J.I., Hammond S. & Caniano D.A. (2004) Clinical manifestations of appendiceal pinworms in children: an institutional experience and a review of the literature. Pediatric Surgery International 20, 372–375. [DOI] [PubMed] [Google Scholar]
- Asaolu S.O., Holland C.V. & Crompton D.W. (1991)Community control of Ascaris lumbricoides in rural Oyo State, Nigeria: mass, targeted and selective treatment with levamisole. Parasitology 103, 291–298. [DOI] [PubMed] [Google Scholar]
- Awasthi S. & Pande V.K. (2001) Six‐monthly de‐worming in infants to study effects on growth. Indian Journal of Pediatrics 68, 823–827. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Pande V.K. & Fletcher R.H. (2000) Effectiveness and cost‐effectiveness of albendazole in improving nutritional status of pre‐school children in urban slums. Indian Pediatrics 37, 19–29. [PubMed] [Google Scholar]
- Beach M.J., Roberts J.M., Prospere R., Streit T.G., Addiss D.G. & Lammie P.J. (1999a) Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian schoolchildren. American Journal of Tropical Medicine and Hygiene 60, 479–486. [DOI] [PubMed] [Google Scholar]
- Beach M.J., Streit T.G., Addiss D.G., Prospere R., Roberts J.M. & Lammie P.J. (1999b) Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian schoolchildren. American Journal of Tropical Medicine and Hygiene 60, 479–486. [DOI] [PubMed] [Google Scholar]
- Beasley N.M., Tomkins A.M., Hall A., Kihamia C.M., Lorri W., Nduma B. et al (1999) The impact of population level deworming on the haemoglobin levels of schoolchildren in Tanga, Tanzania. Tropical Medicine and International Health 4, 744. [DOI] [PubMed] [Google Scholar]
- Beasley N.M., Tomkins A.M., Hall A., Lorri W., Kihamia C.M. & Bundy D.A. (2000) The impact of weekly iron supplementation on the iron status and growth of adolescent girls in Tanzania. Tropical Medicine and International Health 5, 794. [DOI] [PubMed] [Google Scholar]
- Belizario V.Y., Amarillo M.E., De Leon W.U., Reyes A.E., Bugayong M.G. & Macatangay B.J. (2003) A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bulletin of the World Health Organization 81, 35–42. [PMC free article] [PubMed] [Google Scholar]
- Bennett A.G.H. (2000) Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitology Today 16, 71–74. [DOI] [PubMed] [Google Scholar]
- Bhargava A. (2000) Treatment for intestinal helminth infection. Conclusions should have been based on broader considerations. British Medical Journal 321, 1225. [PubMed] [Google Scholar]
- Bhargava A., Jukes M., Lambo J., Kihamia C.M., Lorri W., Nokes C. et al (2003) Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food and Nutrition Bulletin 24, 332–342. [DOI] [PubMed] [Google Scholar]
- Bobak D.A. (2006) Use of nitazoxanide for gastrointestinal tract infections: treatment of protozoan parasitic infection and beyond. Current Infectious Disease Reports 8, 91–95. [DOI] [PubMed] [Google Scholar]
- Boes J., Roepstorff A., Nansen P., Medley G.F. & Eriksen L. (1998) Distribution of Ascaris suum in experimentally and naturally infected pigs and comparison with Ascaris lumbricoides infections in humans. Parasitology 117, 589–596. [DOI] [PubMed] [Google Scholar]
- De Boissieu D., Raymond J., Dupont C., Chaussain M. & Badoual J. (1996) Small‐bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain, or both. Journal of Pediatrics 128, 203–207. [DOI] [PubMed] [Google Scholar]
- Booth M. & Bundy D.A. (1992) Comparative prevalences of Ascaris lumbricoides, Trichuris trichiura and hookworm infections and the prospects for combined control. Parasitology 105, 151–157. [DOI] [PubMed] [Google Scholar]
- Bradley M., Chandiwana S.K., Bundy D.A. & Medley G.F. (1992) The epidemiology and population biology of Necator americanus infection in a rural community in Zimbabwe. Transactions of the Royal Society of Tropical Medicine and Hygiene 86, 73–76. [DOI] [PubMed] [Google Scholar]
- Braun T.I., Fekete T. & Lynch A. (1988) Strongyloidiasis in an institution for mentally retarded adults. Archives of International Medicine 148, 634–636. [PubMed] [Google Scholar]
- Brooker S., Marriot H., Hall A., Adjei S., Allan E., Maier C. et al (2001) Community perception of school‐based delivery of anthelmintics in Ghana and Tanzania. Tropical Medicine and International Health 6, 1075. [DOI] [PubMed] [Google Scholar]
- Bundy D. & Peto R. (2000) Treatment for intestinal helminth infection. Studies of short term treatment cannot assess long term benefits of regular treatment. British Medical Journal 321, 1225. [PubMed] [Google Scholar]
- Bundy D.A. & Cooper E.S. (1988) The evidence for predisposition to trichuriasis in humans: comparison of institutional and community studies. Annals of Tropical Medicine and Parasitology 82, 251–256. [DOI] [PubMed] [Google Scholar]
- Bundy D.A. & Cooper E.S. (1989) Trichuris and trichuriasis in humans. Advances in Parasitology 28, 107–173. [DOI] [PubMed] [Google Scholar]
- Bundy D.A., Hall A., Medley G.F. & Savioli L. (1992a) Evaluating measures to control intestinal parasitic infections. World Health Statistics Quarterly. Rapport Trimestriel de Statistiques Sanitaires Mondiales 45, 168–179. [PubMed] [Google Scholar]
- Bundy D.A., Horton J., Wong M.S. & Lewis L.L. (1992b) Control of geohelminths by delivery of targeted chemotherapy through schools. Transactions of the Royal Society of Tropical Medicine and Hygiene 84, 115–120. [DOI] [PubMed] [Google Scholar]
- Bundy D.A.P. (1990) Is the hookworm just another geohelminth? In: Hookworm Disease. Current Status and New Directions (eds Schad G.A. & Warren K.S.), pp 147–164. Taylor & Francis: London. [Google Scholar]
- Butterworth A.E., Sturrock R.F., Ouma J.H., Mbugua G.G., Fulford A.J., Kariuki H.C. et al (1991) Comparison of different chemotherapy strategies against Schistosoma mansoni in Machakos District, Kenya: effects on human infection and morbidity. Parasitology 103, 339. [DOI] [PubMed] [Google Scholar]
- Cairncross S., Moraes L., Tayeh A., Blumenthal U. & Kolsky P. (1996) The public and domestic domains in the transmission of disease. Tropical Medicine and International Health 1, 27–34. [DOI] [PubMed] [Google Scholar]
- Callender J.E., Grantham‐McGregor S.M., Walker S.P. & Cooper E.S. (1994) Treatment effects in Trichuris dysentery syndrome. Acta Paediatrica 83, 1182–1187. [DOI] [PubMed] [Google Scholar]
- Carney W.P. (1991) Echinostomiasis – a snail‐borne intestinal trematode zoonosis. Southeast Asian Journal of Tropical Medicine and Public Health 22 (Suppl.), 206–211. [PubMed] [Google Scholar]
- Carrera E., Nesheim M.C. & Crompton D.W. (1984) Lactose maldigestion in Ascaris‐infected preschool children. American Journal of Clinical Nutrition 39, 255–264. [DOI] [PubMed] [Google Scholar]
- Celiksoz A., Acioz M., Degerli S., Alim A. & Aygan C. (2005) Egg positive rate of Enterobius vermicularis and Taenia spp. by cellophane tape method in primary school children in Sivas, Turkey. Korean Journal of Parasitology 43, 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf B.J., Rohde J.E. & Soesanto T. (1981) Ascaris and malnutrition in a Balinese village: a conditional relationship. Tropical and Geographical Medicine 33, 367–373. [PubMed] [Google Scholar]
- Chan L., Bundy D.A. & Kan S.P. (1994) Aggregation and predisposition to Ascaris lumbricoides and Trichuris trichiura at the familial level. Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 46–48. [DOI] [PubMed] [Google Scholar]
- Chandra S.S. (1984) Epidemiology of Fasciolopsis buski in Uttar Pradesh. Indian Journal of Medical Research 79, 55–59. [PubMed] [Google Scholar]
- Coles G.C. (1995) Chemotherapy of human nematodes: learning from the problems in sheep. Journal of the Royal Society of Medicine 88, 649P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D.J., Hall A., Anwar K.S., Rahman M.L. & Bundy D.A. (1995) Household aggregation of Strongyloides stercoralis infection in Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 89, 258–261. [DOI] [PubMed] [Google Scholar]
- Coombs I. & Crompton D.W.T. (1991) A Guide to Human Helminths. Taylor and Francis: London. [Google Scholar]
- Cooper E. (2000) Treatment for intestinal helminth infection. Message does not follow from systematic review's findings. British Medical Journal 321, 1225–1226. [PubMed] [Google Scholar]
- Cooper E.S., Bundy D.A., Duff E.M. & Howell S. (1995) Catch‐up’ growth velocities after treatment for Trichuris dysentery syndrome. Transactions of the Royal Society of Tropical Medicine and Hygiene 89, 653. [DOI] [PubMed] [Google Scholar]
- Crompton D.W. & Whitehead R.R. (1993) Hookworm infections and human iron metabolism. Parasitology 107 (Suppl.), S137–S145. [DOI] [PubMed] [Google Scholar]
- Crompton D.W.T. (1989) Biology of Ascaris lumbricoides In: Ascariasis and Its Prevention and Control (eds Crompton D.W.T., Nesheim M.C. & Pawlowski Z.S.), pp 9–44. Taylor and Francis: London. [Google Scholar]
- Dickson R., Demellweek C., Garner P., Awasthi S. & Williamson P. (2000a) Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. British Medical Journal 320, 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R., Williamson P., Awasthi S. & Demellweek C. (2000b) Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance. Cochrane Database of Systematic Reviews 320, CD000371. [DOI] [PubMed] [Google Scholar]
- Dickson R., Awasthi S., Demellweek C. & Williamson P. (2005) Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No. CD000371. doi:10.1002/14651858.CD000371. [DOI] [PubMed] [Google Scholar]
- Donnen P., Brasseur D., Dramaix M., Vertongen F., Zihindula M., Muhamiriza M. et al (1998) Vitamin A supplementation but not deworming improves growth of malnourished preschool children in eastern Zaire. Journal of Nutrition 128, 1320–1327. [DOI] [PubMed] [Google Scholar]
- Dossa R.A., Ategbo E.A., De Koning F.L., Van Raaij J.M. & Hautvast J.G. (2001) Impact of iron supplementation and deworming on growth performance in preschool Beninese children. European Journal of Clinical Nutrition 55, 223–228. [DOI] [PubMed] [Google Scholar]
- Drake L., Korchev Y., Bashford L., Djamgoz M., Wakelin D., Ashall F. et al (1994) The major secreted product of the whipworm, Trichuris, is a pore‐forming protein. Proceeding Biological Sciences 257, 255–261. [DOI] [PubMed] [Google Scholar]
- Easton A. (1999) Intestinal worms impair child health in the Philippines. British Medical Journal 318, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger R.J., Chusilp K., Saowakontha S., Wedel M., Hofhuis E.H., Bloem M.W. et al (1990) Association between intestinal parasitoses and nutritional status in 3‐8‐year‐old children in northeast Thailand. Tropical and Geographical Medicine 42, 312–323. [PubMed] [Google Scholar]
- El‐On J. (2003) Benzimidazole treatment of cystic echinococcosis. Acta Tropica 85, 243–252. [DOI] [PubMed] [Google Scholar]
- Else K.J. (2005) Have gastrointestinal nematodes outwitted the immune system? Parasite Immunology 27, 407–415. [DOI] [PubMed] [Google Scholar]
- Eom K.S. & Rim H.J. (1993) Morphologic descriptions of Taenia asiatica sp. n. Korean Journal of Parasitology 31, 1–6. [DOI] [PubMed] [Google Scholar]
- Esposito M., Stettler R., Moores S.L., Pidathala C., Muller N., Stachulski A. et al (2005) In vitro efficacies of nitazoxanide and other thiazolides against Neospora caninum tachyzoites reveal antiparasitic activity independent of the nitro group. Antimicrobial Agents and Chemotherapy 49, 3715–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J.J. & Prescott C.A. (1993) Ascariasis and acute otitis media. International Journal of Pediatric Otorhinolaryngology 26, 67–69. [DOI] [PubMed] [Google Scholar]
- Farthing M.J. (1993) Diarrhoeal disease: current concepts and future challenges. Pathogenesis of giardiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 87 (Suppl. 3 ), 17–21. [DOI] [PubMed] [Google Scholar]
- Fernando M.A., Balasuriya Xx. & Somaratne Xx. (1983) Effect of Ascaris lumbricoides infestation on growth of children. Indian Pediatrics 20, 721–731. [PubMed] [Google Scholar]
- Ferreyra N.P. & Cerri G.G. (1998) Ascariasis of the alimentary tract, liver, pancreas and biliary system: its diagnosis by ultrasonography. Hepato-gastroenterology 45, 932–937. [PubMed] [Google Scholar]
- Forrester J.E., Golden M.H., Scott M.E. & Bundy D.A. (1990) Predisposition of individuals and families in Mexico to heavy infection with Ascaris lumbricoides and Trichuris trichiura . Transactions of the Royal Society of Tropical Medicine and Hygiene 84, 272–276. [DOI] [PubMed] [Google Scholar]
- Forrester J.E., Bailar J.C. 3rd, Esrey S.A., Jose M.V., Castillejos B.T. & Ocampo G. (1998) Randomised trial of albendazole and pyrantel in symptomless trichuriasis in children. Lancet 352, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Fox L.M., Furness B.W., Haser J.K., Desire D., Brissau J.M., Milord M.D. et al (2005) Tolerance and efficacy of combined diethylcarbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. American Journal of Tropical Medicine and Hygiene 73, 115–121. [PubMed] [Google Scholar]
- Freij L., Meeuwisse G.W., Berg N.O., Wall S., Gebre‐Medhin M. (1979) Ascariasis and malnutrition. A study in urban Ethiopian children. American Journal of Clinical Nutrition 32, 1545–1553. [DOI] [PubMed] [Google Scholar]
- Fried B., Graczyk T.K. & Tamang L. (2004) Food‐borne intestinal trematodiases in humans. Parasitology Research 93, 159–170. [DOI] [PubMed] [Google Scholar]
- Friis H., Mwaniki D., Omondi B., Muniu E., Thiong’o F., Ouma J. et al (2003) Effects on haemoglobin of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 57, 573–579. [DOI] [PubMed] [Google Scholar]
- Gaasenbeek C.P. & Borgsteede F.H. (1998) Studies on the survival of Ascaris suum eggs under laboratory and simulated field conditions. Veterinary Parasitology 75, 227–234. [DOI] [PubMed] [Google Scholar]
- Gajdusek D.C. (1978) Introduction of Taenia solium into west New Guinea with a note on an epidemic of burns from cysticercus epilepsy in the Ekari people of the Wissel Lakes area. Papua New Guinea Medical Journal 21, 329–342. [PubMed] [Google Scholar]
- Garg R., Lee L.A., Beach M.J., Wamae C.N., Ramakrishnan U. & Deming M.S. (2002) Evaluation of the Integrated Management of Childhood Illness guidelines for treatment of intestinal helminth infections among sick children aged 2–4 years in western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 543–548. [DOI] [PubMed] [Google Scholar]
- Gatti S., Lopes R., Cevini C., Ijaoba B., Bruno A., Bernuzzi A.M. et al (2000) Intestinal parasitic infections in an institution for the mentally retarded. Annals of Tropical Medicine and Parasitology 94, 453–460. [DOI] [PubMed] [Google Scholar]
- Geissler P.W., Mwaniki D., Thiong F. & Friis H. (1998) Geophagy as a risk factor for geohelminth infections: a longitudinal study of Kenyan primary schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene 92, 7–11. [DOI] [PubMed] [Google Scholar]
- Gelpi A.P. & Mustafa A. (1968) Ascaris pneumonia. American Journal of Medicine 44, 377–389. [DOI] [PubMed] [Google Scholar]
- Gendrel D., Moreno J.L., Dupont C., Nardou M., Richard‐Lenoble D., Kombila M. et al (1992) Influence of intestinal parasitism on lactose absorption in well‐nourished African children. American Journal of Tropical Medicine and Hygiene 46, 137–140. [DOI] [PubMed] [Google Scholar]
- Gibson R.S. (1990) Principles of Nutritional Assessment. Oxford University Press: Oxford. [Google Scholar]
- Gilgen D.D., Mascie‐Taylor C.G. & Rosetta L.L. (2001) Intestinal helminth infections, anaemia and labour productivity of female tea pluckers in Bangladesh. Tropical Medicine and International Health 6, 449–457. [DOI] [PubMed] [Google Scholar]
- Gill G.V. & Bell D.R. (1979) Strongyloides stercoralis infection in former Far East prisoners of war. British Medical Journal 2, 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G.V. & Bell D.R. (1987) Strongyloides stercoralis infection in Burma Star veterans. British Medical Journal (Clinical Research Ed.) 294, 1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G.V., Bell D.R., Beeching N.J., Welch E. & Bailey J.W. (2004) Chronic Strongyloides stercoralis infection in former British Far East prisoners of war. Quarterly Journal of Medicine 97, 789–795. [DOI] [PubMed] [Google Scholar]
- Gilles H. (1990) Naturally acquired infections: what's needed? In: Hookworm Disease. Current Status and New Directions (eds Schad G.A. & Warren K.S.), 221–230. Taylor and Francis: London. [Google Scholar]
- Gilman R.H., Mondal G., Maksud M., Alam K., Rutherford E., Gilman J.B. et al (1982) Endemic focus of Fasciolopsis buski infection in Bangladesh. American Journal of Tropical Medicine and Hygiene 31, 796–802. [DOI] [PubMed] [Google Scholar]
- Golden M.H. (1994) Is complete catch‐up possible for stunted malnourished children? European Journal of Clinical Nutrition 48 (Suppl. 1 ), S58–S70. [PubMed] [Google Scholar]
- Greenberg B.L., Gilman R.H., Shapiro H., Gilman J.B., Mondal G., Maksud M. et al (1981) Single dose piperazine therapy for Ascaris lumbricoides: an unsuccessful method of promoting growth. American Journal of Clinical Nutrition 34, 2508–2516. [DOI] [PubMed] [Google Scholar]
- Gupta M.C. (1990) Effect of ascariasis upon nutritional status of children. Journal of Tropical Pediatrics 36, 189–191. [DOI] [PubMed] [Google Scholar]
- Gupta M.C. & Urrutia J.J. (1982) Effect of periodic antiascaris and antigiardia treatment on nutritional status of preschool children. American Journal of Clinical Nutrition 36, 79–86. [DOI] [PubMed] [Google Scholar]
- Gustafsson L.L., Beerman B. & Abdi Y.A. (1987) Handbook of Drugs for Tropical Parasitic Infections. Taylor and Francis: London. [Google Scholar]
- Guyatt H.L., Bundy D.A., Medley G.F. & Grenfell B.T. (1990) The relationship between the frequency distribution of Ascaris lumbricoides and the prevalence and intensity of infection in human communities. Parasitology 101, 139–143. [DOI] [PubMed] [Google Scholar]
- Hadidjaja P., Abidin S.A., Ismid I.S., Bonang E., Suyardi M.A. & Margono S.S. (1998) The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides . American Journal of Tropical Medicine and Hygiene 59, 791–795. [DOI] [PubMed] [Google Scholar]
- Hadju V., Stephenson L., Abadi K., Mohammed H., Bowman D. & Parker R. (1996) Improvements in appetite and growth in helminth‐infected schoolboys three and seven weeks after a single dose of pyrantel pamoate. Parasitology 113, 497–504. [DOI] [PubMed] [Google Scholar]
- Hall A. (1981) Quantitative variability of nematode egg counts in faeces: a study among rural Kenyans. Transactions of the Royal Society of Tropical Medicine and Hygiene 75, 682–687. [DOI] [PubMed] [Google Scholar]
- Hall A. (1983) Dietary protein and the growth of rats infected with the tapeworm Hymenolepis diminuta . British Journal of Nutrition 49, 59–65. [DOI] [PubMed] [Google Scholar]
- Hall A. (1985) Nutritional aspects of parasitic infection. Progress in Food and Nutrition Science 9, 227–256. [PubMed] [Google Scholar]
- Hall A. (2007) Micronutrient supplements for children after deworming. Lancet Infectious Diseases 7, 297–302. [DOI] [PubMed] [Google Scholar]
- Hall A. & Holland C. (2000) Geographical variation in Ascaris lumbricoides fecundity and its implications for helminth control. Parasitology Today 16, 540–544. [DOI] [PubMed] [Google Scholar]
- Hall A. & Nahar Q. (1994) Albendazole and infections with Ascaris lumbricoides and Trichuris trichiura in children in Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 110–112. [DOI] [PubMed] [Google Scholar]
- Hall A. & Romanova T. (1990) Ascaris lumbricoides: detecting its metabolites in the urine of infected people using gas‐liquid chromatography. Experimental Parasitology 70, 35–42. [DOI] [PubMed] [Google Scholar]
- Hall A., Latham M.C., Crompton D.W. & Stephenson L.S. (1981) Taenia saginata (Cestoda) in western Kenya: the reliability of faecal examinations in diagnosis. Parasitology 83, 91–101. [DOI] [PubMed] [Google Scholar]
- Hall A., Anwar K.S. & Tomkins A.M. (1992) Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. Lancet 339, 1253–1257. [DOI] [PubMed] [Google Scholar]
- Hall A., Conway D.J., Anwar K.S. & Rahman M.L. (1994) Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 527–530. [DOI] [PubMed] [Google Scholar]
- Hall A., Adjei S. & Kihamia C. (1996) School health programmes. Africa Health 18, 22–23. [PubMed] [Google Scholar]
- Hall A., Anwar K.S., Tomkins A. & Rahman L. (1999) The distribution of Ascaris lumbricoides in human hosts: a study of 1765 people in Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 93, 503–510. [DOI] [PubMed] [Google Scholar]
- Hall A., Bobrow E., Brooker S., Jukes M., Nokes K., Lambo J. et al (2001) Anaemia in schoolchildren in eight countries in Africa and Asia. Public Health Nutrition 4, 749. [DOI] [PubMed] [Google Scholar]
- Hall A., Roschnik N., Ouattara F., Toure I., Maiga F., Sacko M. et al (2002) A randomised trial in Mali of the effectiveness of weekly iron supplements given by teachers on the haemoglobin concentrations of schoolchildren. Public Health Nutrition 5, 413–418. [DOI] [PubMed] [Google Scholar]
- Hall A., Hahn T.T.M., Farley K., Quynh T.P.N. & Valdivia F. (2007) An evaluation of the impact of a school nutrition programme in Vietnam. Public Health Nutrition 10, 819–826. [DOI] [PubMed] [Google Scholar]
- Haswell‐Elkins M.R., Elkins D.B. & Anderson R.M. (1987) Evidence for predisposition in humans to infection with Ascaris, hookworm, Enterobius and Trichuris in a South Indian fishing community. Parasitology 95, 323–337. [DOI] [PubMed] [Google Scholar]
- Hautus M.A., Kortbeek L.M., Vetter J.C. & Laarman J.J. (1988) In vitro excystation and subsequent axenic growth of Giardia lamblia . Transactions of the Royal Society of Tropical Medicine and Hygiene 82, 858–861. [DOI] [PubMed] [Google Scholar]
- Holland C.V., Macdonald R., Stoddart R.C., Asaolu S.O., Crompton D.W. & Torimiro S.E. (1989) The epidemiology of Ascaris lumbricoides and other soil‐transmitted helminths in primary school children from Ile‐Ife, Nigeria. Parasitology 99, 275–285. [DOI] [PubMed] [Google Scholar]
- Horton J. (2000) Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 121 (Suppl.), S113–S132. [DOI] [PubMed] [Google Scholar]
- Horton J. (2002) Albendazole: a broad spectrum anthelminthic for treatment of individuals and populations. Current Opinion in Infectious Diseases 15, 599–608. [DOI] [PubMed] [Google Scholar]
- Horton J. (2003a) Albendazole for the treatment of echinococcosis. Fundamental and Clinical Pharmacology 17, 205–212. [DOI] [PubMed] [Google Scholar]
- Horton J. (2003b) Global anthelmintic chemotherapy programs: learning from history. Trends in Parasitology 19, 405–409. [DOI] [PubMed] [Google Scholar]
- Horton J., Addiss D.G., Dunyo S.K., Lazdins J.K., Beach M.J., Witt C. et al (2000) An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis. Parasitology 121 (Suppl.), S147–S160. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. & Cerami A. (1983) Secretion of a proteolytic anticoagulant by Ancylostoma hookworms. Journal of Experimental Medicine 157, 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman J.E. & Fried B. (1990) Echinostoma and echinostomiasis. Advances in Parasitology 29, 215–269. [DOI] [PubMed] [Google Scholar]
- Hunt J.M. (2002) Reversing productivity losses from iron deficiency: the economic case. Journal of Nutrition 132, S794–S801. [DOI] [PubMed] [Google Scholar]
- Huq F., Asaduzzaman M., Yasmin M., Hamid A.A. & Ali S. (1982) Epidemiological study and comparison of pyrantel and levamisole in the treatment of roundworm and hookworm infestations. Bangladesh Medical Research Council Bulletin 8, 1–6. [PubMed] [Google Scholar]
- Jain K.C. & Pahuja O.P. (1988) Extension of round worm through ear. Indian Pediatrics 25, 104–105. [PubMed] [Google Scholar]
- Jalal F., Sanjur D., Habicht J.P., Nesheim M.C. & Agus Z. (1998) Serum retinol concentrations in children are affected by food sources of beta‐carotene, fat intake, and anthelmintic drug treatment. American Journal of Clinical Nutrition 68, 623–629. [DOI] [PubMed] [Google Scholar]
- Jinabhai C.C., Taylor M., Coutsoudis A., Coovadia H.M., Tomkins A.M. & Sullivan K.R. (2001a) Epidemiology of helminth infections: implications for parasite control programmes, a South African perspective. Public Health Nutrition 4, 1211–1219. [DOI] [PubMed] [Google Scholar]
- Jinabhai C.C., Taylor M., Coutsoudis A., Coovadia H.M., Tomkins A.M. & Sullivan K.R. (2001b) A randomized controlled trial of the effect of antihelminthic treatment and micronutrient fortification on health status and school performance of rural primary school children. Annals of Tropical Paediatrics 21, 319–333. [DOI] [PubMed] [Google Scholar]
- Juan J.O., Lopez Chegne N., Gargala G. & Favennec L. (2002) Comparative clinical studies of nitazoxanide, albendazole and praziquantel in the treatment of ascariasis, trichuriasis and hymenolepiasis in children from Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 193–196. [DOI] [PubMed] [Google Scholar]
- Jukes M.C.H., Kihamia C., Yona E., Lambo J.K., Mbise A., Nokes C.A. et al (2002) Heavy schistosomiasis associated with poor short‐term memory and slower reaction times in Tanzanian schoolchildren. Tropical Medicine and International Health 7, 104–117. [DOI] [PubMed] [Google Scholar]
- Kapil U. (2002) Deaths in Assam during vitamin A pulse distribution: the needle of suspicion is on the new measuring cup. Indian Pediatrics 39, 114–115. [PubMed] [Google Scholar]
- Kapil U. (2004) Update on vitamin A‐related deaths in Assam, India. American Journal of Clinical Nutrition 80, 1082–1084. [DOI] [PubMed] [Google Scholar]
- Karyadi E., Gross R., Sastroamidjojo S., Dillon D., Richards A.L. & Sutanto I. (1996) Anthelminthic treatment raises plasma iron levels but does not decrease the acute‐phase response in Jakarta school children. Southeast Asian Journal of Tropical Medicine and Public Health 27, 742–753. [PubMed] [Google Scholar]
- Keiser P.B. & Nutman T.B. (2004) Strongyloides stercoralis in the immunocompromised population. Clinical Microbiology Reviews 17, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H.M., Shimi S., Sarwat M.A., Fawzy A.F. & Sorougy A.O. (1991) Recent study of Hymenolepis nana infection in Egyptian children. Journal of the Egyptian Society of Parasitology 21, 293–300. [PubMed] [Google Scholar]
- Khin Maung U., Bolin T.D., Duncombe V.M., Pereira S.P., Myo K., Nyunt Nyunt W. et al (1990) Effect of short‐term intermittent antibiotic treatment on growth of Burmese (Myanmar) village children. Lancet 336, 1090–1093. [DOI] [PubMed] [Google Scholar]
- Kightlinger L.K., Seed J.R. & Kightlinger M.B. (1995) The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. Journal of Parasitology 81, 159–169. [PubMed] [Google Scholar]
- Kilama W.L. (1989) Sanitation in the control of ascariasis In: Ascariasis and Its Prevention and Control (eds Crompton D.W.T., Nesheim M.C. & Pawlowski Z.S.), pp 289–300. Taylor and Francis: London. [Google Scholar]
- Kloetzel K., Merluzzi Filho T.J. & Kloetzel D. (1982) Ascaris and malnutrition in a group of Brazilian children – a follow‐up study. Journal of Tropical Pediatrics 28, 41–43. [DOI] [PubMed] [Google Scholar]
- Kofie B.A. & Dipeolu O.O. (1983) A study of human and porcine Ascariasis in a rural area of South‐West Nigeria. International Journal of Zoonoses 10, 66–70. [PubMed] [Google Scholar]
- Koroma M.M., Williams R.A., Haye R.R. & Hodges M. (1996) Effects of albendazole on growth of primary school children and the prevalence and intensity of soil‐transmitted helminths in Sierra Leone. Journal of Tropical Pediatrics 42, 371–372. [DOI] [PubMed] [Google Scholar]
- Kruger M., Badenhorst C., Mansvelt E., Laubscher J. & Benade S.A. (1996) Effects of iron fortification in a school feeding scheme and anthelmintic therapy on the iron status and growth of six‐ to eight‐year‐old schoolchildren. Food and Nutrition Bulletin 17, 11–21. [Google Scholar]
- Kurz K.M., Stephenson L.S., Latham M.C. & Kinoti S.N. (1986) The effectiveness of metrifonate in reducing hookworm infection in Kenyan school children. American Journal of Tropical Medicine and Hygiene 35, 571–574. [DOI] [PubMed] [Google Scholar]
- Lai K.P., Kaur H., Mathias R.G. & Ow‐Yang C.K. (1995) Ascaris and Trichuris do not contribute to growth retardation in primary school children. Southeast Asian Journal of Tropical Medicine and Public Health 26, 322–328. [PubMed] [Google Scholar]
- Latham M.C., Stephenson L.S., Kinoti S.N., Zaman M.S. & Kurz K.M. (1990a) Improvements in growth following iron supplementation in young Kenyan school children. Nutrition 6, 159–165. [PubMed] [Google Scholar]
- Latham M.C., Stephenson L.S., Kurz K.M. & Kinoti S.N. (1990b) Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. American Journal of Tropical Medicine and Hygiene 43, 170. [DOI] [PubMed] [Google Scholar]
- Lind T., Gamayanti I.L., Persson L‐A, Ismail D., Lnnerdal B., Stenlund H. et al (2004) A community‐based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. American Journal of Clinical Nutrition 80, 729–736. [DOI] [PubMed] [Google Scholar]
- Lwambo N.J., Bundy D.A. & Medley G.F. (1992) A new approach to morbidity risk assessment in hookworm endemic communities. Epidemiology and Infection 108, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabaso M.L.H., Gouws E., Appleton C.C. & Hughes J.C. (2003) The effect of soil type and climate on hookworm (Necator americanus) distribution in KwaZulu‐Natal, South Africa. Tropical Medicine and International Health 8, 722–727. [DOI] [PubMed] [Google Scholar]
- Macpherson C.N. (2005) Human behaviour and the epidemiology of parasitic zoonoses. International Journal for Parasitology 35, 1319–1331. [DOI] [PubMed] [Google Scholar]
- Mahendra Raj S. (1998) Intestinal geohelminthiasis and growth in pre‐adolescent primary school children in Northeastern Peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 29, 112–117. [PubMed] [Google Scholar]
- Mahendra Raj S. & Naing N.N. (1998) Ascariasis, trichuriasis, and growth of schoolchildren in Northeastern Peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 29, 729–734. [PubMed] [Google Scholar]
- Mahendra Raj S., Mustaffa B.E., Sein K.T. & Anuar K.A. (1997a) Intestinal helminthiasis in relation to height and weight of early primary school children in northeastern peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 28, 314–320. [PubMed] [Google Scholar]
- Mahendra Raj S., Sein K.T., Khairul Anuar A. & Mustaffa B.E. (1997b) Intestinal helminthiasis in relation to height and weight of early primary school children in northeastern peninsular Malaysia. Southeast Asian Journal of Tropical Medicine Public Health 28, 314–320. [PubMed] [Google Scholar]
- Mahomed A.A., MacKenzie R.N., Carson L.S. & Jibril J.A. (2003) Enterobius vermicularis and perianal sepsis in children. Pediatric Surgery International 19, 740–741. [DOI] [PubMed] [Google Scholar]
- Majumdar I., Paul P., Talib V.H. & Ranga S. (2003) The effect of iron therapy on the growth of iron‐replete and iron‐deplete children. Journal of Tropical Pediatrics 49, 84–88. [DOI] [PubMed] [Google Scholar]
- Marinho H.A., Shrimpton R., Giugliano R. & Burini R.C. (1991) Influence of enteral parasites on the blood vitamin A levels in preschool children orally supplemented with retinol and/or zinc. European Journal of Clinical Nutrition 45, 539–544. [PubMed] [Google Scholar]
- Martin J., Nesheim M.C., Crompton D.W. & Carrera E. (1984) Mucosal surface lesions in young protein‐deficient pigs infected with Ascaris suum (Nematoda). Parasitology 88, 333–340. [PubMed] [Google Scholar]
- Maruyama H., Mimori T., Nawa Y. & Noda S. (1997) An outbreak of ascariasis with marked eosinophilia in the southern part of Kyushu District, Japan, caused by infection with swine Ascaris . Southeast Asian Journal of Tropical Medicine and Public Health 28 (Suppl. 1), 194–196. [PubMed] [Google Scholar]
- Mas‐Coma S., Bargues M.D. & Valero M.A. (2005) Fascioliasis and other plant‐borne trematode zoonoses. International Journal for Parasitology 35, 1255–1278. [DOI] [PubMed] [Google Scholar]
- Mason P.R. & Patterson B.A. (1994) Epidemiology of Hymenolepis nana infections in primary school children in urban and rural communities in Zimbabwe. Journal of Parasitology 80, 245–250. [PubMed] [Google Scholar]
- Mata L.J. (1978) The Children of Santa Mara Cauqué. MIT Press: Cambridge, MA. [Google Scholar]
- Megraud F., Occhialini A. & Rossignol J.F. (1998) Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross‐resistance to metronidazole. Antimicrobial Agents and Chemotherapy 42, 2836–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael E. (2000) Treatment for intestinal helminth infection. Contrary to authors’ comments, meta‐analysis supports global helminth control initiatives. British Medical Journal 321, 1224–1225. [PMC free article] [PubMed] [Google Scholar]
- Michaelsen K.F. (1985) Hookworm infection in Kweneng District, Botswana, a prevalence survey and a controlled treatment trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 79, 848. [DOI] [PubMed] [Google Scholar]
- Moore S.R., Schorling J.B., Soares A.M., Lima A.A., Conaway M.R. & Guerrant R.L. (2001) Early childhood diarrhoea and helminthiases associate with long‐term linear growth faltering. International Journal of Epidemiology 30, 1457–1464. [DOI] [PubMed] [Google Scholar]
- Muniz P.T., Conde W.L., Monteiro C.A., Ferreira M.U. & Ferreira C.S. (2002) Intestinal parasitic infections in young children in São Paulo, Brazil: prevalences, temporal trends and associations with physical growth. Annals of Tropical Medicine and Parasitology 96, 503–512. [DOI] [PubMed] [Google Scholar]
- Mwaniki D., Omondi B., Muniu E., Thiong’o F., Ouma J., Magnussen P. et al (2002) Effects on serum retinol of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomised, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 56, 666–673. [DOI] [PubMed] [Google Scholar]
- Mwanri L., Worsley A., Ryan P., Masika J. (2000) Supplemental vitamin A improves anemia and growth in anemic school children in Tanzania. Journal of Nutrition 130, 2691–2696. [DOI] [PubMed] [Google Scholar]
- Müller N. & Von Allmen N. (2005) Recent insights into the mucosal reactions associated with Giardia lamblia infections. International Journal for Parasitology 35, 1339–1347. [DOI] [PubMed] [Google Scholar]
- Needham C., Kim H.T., Hoa N.V., Cong L.D., Michael E., Drake L. et al (1998) Epidemiology of soil‐transmitted nematode infections in Ha Nam Province, Vietnam. Tropical Medicine and International Health 3, 904–912. [DOI] [PubMed] [Google Scholar]
- Newell E., Vyungimana F., Geerts S., Van Kerckhoven I., Tsang V.C. & Engels D. (1997) Prevalence of cysticercosis in epileptics and members of their families in Burundi. Transactions of the Royal Society of Tropical Medicine and Hygiene 91, 389–391. [DOI] [PubMed] [Google Scholar]
- Nokes C., Cooper E.S., Bundy D.A., Grantham‐McGregor S.M. & Sawyer A.W. (1992a) Parasitic helminth infection and cognitive function in school children. Proceedings of the Biology Science 247, 77–81. [DOI] [PubMed] [Google Scholar]
- Nokes C., Cooper E.S., Robinson B.A., Grantham‐McGregor S.M., Sawyer A.W. & Bundy D.A. (1992b) Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology 104, 539–547. [DOI] [PubMed] [Google Scholar]
- Northrop‐Clewes C.A., Lunn P.G., Rousham E.K. & Mascie‐Taylor C.N. (2001) Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. American Journal of Clinical Nutrition 73, 53–60. [DOI] [PubMed] [Google Scholar]
- Nyberg W. (1963) Diphyllobothrium latum and human nutrition, with particular reference to vitamin B12 deficiency. Proceedings of the Nutrition Society 22, 8–14. [DOI] [PubMed] [Google Scholar]
- Olds G.R., King C., Hewlett J., Olveda R., Wu G., Ouma J. et al (1999) Double‐blind placebo‐controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. Journal of Infectious Diseases 179, 996–1003. [DOI] [PubMed] [Google Scholar]
- Olsen A., Thiong’o F.W., Ouma J.H., Mwaniki D., Magnussen P., Michaelsen K.F. et al (2003) Effects of multimicronutrient supplementation on helminth reinfection: a randomized, controlled trial in Kenyan schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene 97, 109–114. [DOI] [PubMed] [Google Scholar]
- Otu‐Bassey I.B., Ejezie G.C., Epoke J. & Useh M.F. (2005) Enterobiasis and its relationship with anal itching and enuresis among school‐age children in Calabar, Nigeria. Annals of Tropical Medicine and Parasitology 99, 611–616. [DOI] [PubMed] [Google Scholar]
- Palupi L., Gross R., Schultink W. & Achadi E. (1997) Effective community intervention to improve hemoglobin status in preschoolers receiving once‐weekly iron supplementation. American Journal of Clinical Nutrition 65, 1057–1061. [DOI] [PubMed] [Google Scholar]
- Pan C.T., Ritchie L.S. & Hunter G.W. (1954) Reinfection and seasonal fluctuations of Ascaris lumbricoides among a group of children in an area where night soil is used. Journal of Parasitology 40, 603–608. [PubMed] [Google Scholar]
- Partnership for Child Development (1998a) Cost of school‐based drug treatment in Tanzania. Health Policy and Planning 14, 384–396. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development (1998b) Implications for school‐based health programmes of age and gender patterns in the Tanzanian primary school. Tropical Medicine and International Health 3, 850–853. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development (1999a) The cost of large‐scale school health programmes which deliver anthelmintics to children in Ghana and Tanzania. Acta Tropica 73, 183–204. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development (1999b) Short stature and the age of enrolment in Primary School: studies in two African countries. Social Science and Medicine 48, 675–682. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development (2001) A Randomised Cluster Trial of Six‐monthly Deworming and Its Effects on the Growth and Educational Achievements of Vietnamese Schoolchildren (unpublished). Partnership for Child Development: Oxford. [Google Scholar]
- Pawlowski Z. & Schultz M.G. (1972) Taeniasis and cysticercosis (Taenia saginata). Advances in Parasitology 10, 269–343. [DOI] [PubMed] [Google Scholar]
- Pawlowski Z.S. & Arfaa F. (1984) Ascariasis In: Tropical and Geographical Medicine (eds Warren K.S. & Mahmoud A.A.F.), pp 347–358. McGraw‐Hill: New York. [Google Scholar]
- Pawlowski Z.S., Schad G.A. & Stot G.J. (1991) Hookworm Infection and Anaemia. Approaches to Prevention and Control. World Health Organization: Geneva. [Google Scholar]
- Pegelow K., Gross R., Pietrzik K., Lukito W., Richards A.L. & Fryauff D.J. (1997) Parasitological and nutritional situation of school children in the Sukaraja district, West Java, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health 28, 173. [PubMed] [Google Scholar]
- Pritchard D.I., McKean P.G., Dale D.D., Slater A.F., Quinnell R.J., Moustafa M. et al (1991) Hookworm (Necator americanus) infection and storage iron depletion. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 235–238. [DOI] [PubMed] [Google Scholar]
- Raisanen S. & Puska P. (1984) Fish tapeworm, a disappearing health problem in Finland. Scandinavian Journal of Social Medicine 12, 3–5. [DOI] [PubMed] [Google Scholar]
- Remm M. (2006) Distribution of enterobiasis among nursery school children in SE Estonia and of other helminthiases in Estonia. Parasitology Research 99, 729–736. [DOI] [PubMed] [Google Scholar]
- RevMan (2003) Review Manager, 4.2 for Windows edn. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen. [Google Scholar]
- Rim H., Lim J., Lee S. & Won C. (1975) Anthelmintic effect of oxantel pamoate and pyrantel pamoate suspension against intestinal nematode infestations. Kisaengchunghak Chapchi. Korean Journal of Parasitology 13, 97–101. [DOI] [PubMed] [Google Scholar]
- Roche M. & Layrisse M. (1966) The nature and causes of ‘hookworm anemia. American Journal of Tropical Medicine and Hygiene 15, 1029–1102. [PubMed] [Google Scholar]
- Romero Cabello R., Guerrero L.R., Munoz Garcia M.R. & Cruz G.A. (1997) Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Transactions of the Royal Society of Tropical Medicine and Hygiene 91, 701–703. [DOI] [PubMed] [Google Scholar]
- Roschnik N., Parawan A., Baylon M.A., Chua T. & Hall A. (2004) Weekly iron supplements given by teachers sustain the haemoglobin concentration of school children in the Philippines. Tropical Medicine and International Health 9, 904–909. [DOI] [PubMed] [Google Scholar]
- Rousham E.K. & Mascie‐Taylor C.G. (1994) An 18‐month study of the effect of periodic anthelminthic treatment on the growth and nutritional status of pre‐school children in Bangladesh. Annals of Human Biology 21, 315–324. [DOI] [PubMed] [Google Scholar]
- Sakti H., Nokes C., Hertanto W.S., Hendratno S., Hall A. & Bundy D.A. (1999) Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Tropical Medicine and International Health 4, 322–334. [DOI] [PubMed] [Google Scholar]
- Salafsky B., Fusco A.C. & Siddiqui A. (1990) Necator americanus: factors influencing skin penetration by larvae In: Hookworm Disease. Current Status and New Directions (eds Schad G.A. & Warren K.S.), pp 329–339. Taylor and Francis: London. [Google Scholar]
- Saldiva S.R., Torres D.M., Silva R.M., Mangini A.C., Silveira A.S., Philippi S.T. et al (1999) Ascaris–Trichuris association and malnutrition in Brazilian children. Paediatric and Perinatal Epidemiology 13, 89–98. [DOI] [PubMed] [Google Scholar]
- Sandars D.F. (1951) Viability of Ascaris lumbricoides eggs preserved in formalin. Nature 167, 730. [DOI] [PubMed] [Google Scholar]
- Sandouk F., Anand B.S., Graham D.Y., Haffar S. & Zada M.M. (1997) Pancreatic‐biliary ascariasis: experience of 300 cases. American Journal of Gastroenterology 92, 2264–2267. [PubMed] [Google Scholar]
- Sarker R.N., Anwar K.S., Biswas K.B. & Mannan M.A. (2002) Effect of deworming on nutritional status of Ascaris infested slum children of Dhaka, Bangladesh. Indian Pediatrics 39, 1021–1026. [PubMed] [Google Scholar]
- Savioli L., Neira M., Albonico M., Beach M.J., Chwaya H.M., Crompton D.W. et al (2000) Treatment for intestinal helminth infection. Review needed to take account of all relevant evidence, not only effects on growth and cognitive performance. British Medical Journal 321, 1226–1227. [PubMed] [Google Scholar]
- Schad G.A. (1989) Morphology and life history of Strongyloides stercoralis In: Strongyloidiasis. A Major Roundworm Infection of Man (ed. Grove D.I.), pp 85–104. Taylor and Francis: London. [Google Scholar]
- Schad G.A. (1990) Hypobiosis and related phenomena in hookworm infection In: Hookworm Disease. Current status and New Directions (eds Schad G.A. & Warren K.S.), pp 71–88. Taylor and Francis: London. [Google Scholar]
- Schaeffer M.W., Buell J.F., Gupta M., Conway G.D., Akhter S.A. & Wagoner L.E. (2004) Strongyloides hyperinfection syndrome after heart transplantation: case report and review of the literature. Journal of Heart and Lung Transplantation 23, 905–911. [DOI] [PubMed] [Google Scholar]
- SC/UK (2004) Emergency Nutrition Assessment. Save the Children, UK: London. [Google Scholar]
- SC/US (2007) A National Survey of the Health of School Children in Ethiopia. Save the Children, USA: Addis Ababa. [Google Scholar]
- Selvaratnam R.R., De Silva N.R., De Silva L.D. & Pathmeswaran A. (2003) Nutritional status and productivity of Sri Lankan tea pluckers. Ceylon Medical Journal 48, 114–118. [DOI] [PubMed] [Google Scholar]
- De Silva N., Guyatt H. & Bundy D. (1997) Anthelmintics. A comparative review of their clinical pharmacology. Drugs 53, 769–788. [DOI] [PubMed] [Google Scholar]
- De Silva N.R., Sirisena J.L., Gunasekera D.P., Ismail M.M. & De Silva H.J. (1999) Effect of mebendazole therapy during pregnancy on birth outcome. Lancet 353, 1145–1149. [DOI] [PubMed] [Google Scholar]
- De Silva N.R., Brooker S., Hotez P.J., Montresor A., Engels D. & Savioli L. (2003) Soil‐transmitted helminth infections: updating the global picture. Trends in Parasitology 19, 547–551. [DOI] [PubMed] [Google Scholar]
- Simeon D.T., Grantham‐McGregor S.M., Callender J.E. & Wong M.S. (1995) Treatment of Trichuris trichiura infections improves growth, spelling scores and school attendance in some children. Journal of Nutrition 125, 1875–1883. [DOI] [PubMed] [Google Scholar]
- Sinniah B. (1982) Daily egg production of Ascaris lumbricoides: the distribution of eggs in the faeces and the variability of egg counts. Parasitology 84, 167–175. [DOI] [PubMed] [Google Scholar]
- Sirivichayakul C., Radomyos P., Praevanit R., Pojjaroen‐Anant C. & Wisetsing P. (2000) Hymenolepis nana infection in Thai children. Journal of the Medical Association of Thailand 83, 1035–1038. [PubMed] [Google Scholar]
- Sisson G., Goodwin A., Raudonikiene A., Hughes N.J., Mukhopadhyay A.K., Berg D.E. et al (2002) Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori . Antimicrobial Agents and Chemotherapy 46, 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. (1990) The ecology of the free‐living stages: a reappraisal In: Hookworm Disease. Current Status and New Directions (eds Schad G.A. & Warren K.S.), pp 89–104. Taylor and Francis: London. [Google Scholar]
- Song H.J., Cho C.H., Kim J.S., Choi M.H. & Hong S.T. (2003) Prevalence and risk factors for enterobiasis among preschool children in a metropolitan city in Korea. Parasitology Research 91, 46–50. [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Jabbar S., Radhakrishna S. & Ramanathan A.M. (1987) Hookworm infection in a rural community in south India and its association with haemoglobin levels. Transactions of the Royal Society of Tropical Medicine and Hygiene 81, 973–977. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S. (1987) Impact of Helminth Infections on Human Nutrition. Taylor and Francis: London. [Google Scholar]
- Stephenson L.S., Krook L.P., Crompton D.W., Pond W.G. & Nesheim M.C. (1980a) Ascaris suum: nutrient absorption, growth, and intestinal pathology in young pigs experimentally infected with 15‐day‐old larvae. Experimental Parasitology 49, 15–25. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Schulpen T.W., Nesheim M.C., Crompton D.W., Latham M.C. & Jansen A.A. (1980b) Relationships between Ascaris infection and growth of malnourished preschool children in Kenya. American Journal of Clinical Nutrition 33, 1165–1172. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Kurz K.M., Kinoti S.N., Oduori M.L. & Crompton D.W. (1985) Relationships of Schistosoma hematobium, hookworm and malarial infections and metrifonate treatment to hemoglobin level in Kenyan school children. American Journal of Tropical Medicine and Hygiene 34, 519. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Kinoti S.N., Latham M.C., Kurz K.M. & Kyobe J. (1989a) Single dose metrifonate or praziquantel treatment in Kenyan children. I. Effects on Schistosoma haematobium, hookworm, hemoglobin levels, splenomegaly, and hepatomegaly. American Journal of Tropical Medicine and Hygiene 41, 436–444. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Kurz K.M. & Kinoti S.N. (1989b) Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. American Journal of Tropical Medicine and Hygiene 41, 445–453. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Kurz K.M., Kinoti S.N. & Brigham H. (1989c) Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. American Journal of Tropical Medicine and Hygiene 41, 78–87. [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Kinoti S.N., Kurz K.M. & Brigham H. (1990) Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Transactions of the Royal Society of Tropical Medicine and Hygiene 84, 277–282. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Adams E.J., Kinoti S.N. & Pertet A. (1993a) Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. Journal of Nutrition 123, 1036–1046. [DOI] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Adams E.J., Kinoti S.N. & Pertet A. (1993b) Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once‐ or twice‐yearly treatment with albendazole. Journal of Nutrition 123, 656–665. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Albonico M., Tielsch J.M., Chwaya H.M. & Savioli L. (1997) School‐based deworming program yields small improvement in growth of Zanzibari school children after one year. Journal of Nutrition 127, 2187–2193. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Chway H.M., Montresor A., Tielsch J.M., Jape J.K., Albonico M. et al (2004) Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. Journal of Nutrition 134, 348–356. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Tielsch J.M., Schulze K.J., Albonico M., Chwaya H.M. & Savioli L. (1998) Effects of the Zanzibar school‐based deworming program on iron status of children. American Journal of Clinical Nutrition 68, 179–186. [DOI] [PubMed] [Google Scholar]
- Sur D., Saha D.R., Manna B., Rajendran K. & Bhattacharya S.K. (2005) Periodic deworming with albendazole and its impact on growth status and diarrhoeal incidence among children in an urban slum of India. Transactions of the Royal Society of Tropical Medicine and Hygiene 99, 261–267. [DOI] [PubMed] [Google Scholar]
- Symons L.E. (1985) Anorexia: occurrence, pathophysiology, and possible causes in parasitic infections. Advances in Parasitology 24, 103–133. [DOI] [PubMed] [Google Scholar]
- Tandon B.N., Tandon R.K. & Satpathy B.K. (1977) Mechanism of malabsorption in giardiasis: a study of bacterial flora and bile salt deconjugation in upper jejunum. Gut 18, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanumihardjo S.A. & Permaesih D. (2004) Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. European Journal of Clinical Nutrition 58, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Tanumihardjo S.A., Muherdiyantiningsih E., Rustan D., Cheng J.C., Permaesih D. & Muhilal J.A. (1996a) Refinement of the modified‐relative‐dose‐response test as a method for assessing vitamin A status in a field setting: experience with Indonesian children. American Journal of Clinical Nutrition 64, 966–971. [DOI] [PubMed] [Google Scholar]
- Tanumihardjo S.A., Permaesih D., Muherdiyantiningsih Xx., Rustan E., Rusmil K., Fatah A.C. et al (1996b) Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. Journal of Nutrition 126, 451–457. [DOI] [PubMed] [Google Scholar]
- Taylor‐Robinson D.C., Jones A.P. & Garner P. (2007) Deworming drugs for treating soil‐transmitted intestinal worms in children: effects on growth and school performance. Cochrane Database of Systematic Reviews 17, CD000371. [DOI] [PubMed] [Google Scholar]
- Thein‐Hlaing, Thane‐Toe, Than‐Saw, Myat‐Lay‐Kyin, Myint‐Lwin (1991) A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 523–528. [DOI] [PubMed] [Google Scholar]
- Todd J., Mosha F., Balira R., Obasi A.I.N., Changalucha J., Ross D.A. et al (2004) The sexual health of pupils in years 4 to 6 of primary schools in rural Tanzania. Sexually Transmitted Infections 80, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashefski J.F., Butler T. & Islam M. (1989) Histopathology and etiology of childhood pneumonia: an autopsy study of 93 patients in Bangladesh. Pathology 21, 71–78. [DOI] [PubMed] [Google Scholar]
- Torlesse H. & Hodges M. (2000) Anthelminthic treatment and haemoglobin concentrations during pregnancy. Lancet 356, 1083. [DOI] [PubMed] [Google Scholar]
- Torlesse H. & Hodges M. (2001) Albendazole therapy and reduced decline in haemoglobin concentration during pregnancy (Sierra Leone). Transactions of the Royal Society of Tropical Medicine and Hygiene 95, 195–201. [DOI] [PubMed] [Google Scholar]
- Al‐Tukhi MH, Al‐Ahdal, M.N. & Peters W. (1991) A simple method for excystation of Giardia lamblia cysts. Annals of Tropical Medicine and Parasitology 85, 427–431. [DOI] [PubMed] [Google Scholar]
- Uglem G.L. & Just J.J. (1983) Trypsin inhibition by tapeworms: antienzyme secretion or pH adjustment? Science 220, 79–81. [DOI] [PubMed] [Google Scholar]
- Valentine C.C., Hoffner R.J. & Henderson S.O. (2001) Three common presentations of ascariasis infection in an urban Emergency Department. Journal of Emergency Medicine 20, 135–139. [DOI] [PubMed] [Google Scholar]
- Waikagul J. (1991) Intestinal fluke infections in Southeast Asia. Southeast Asian Journal of Tropical Medicine and Public Health 22 (Suppl.), 158–162. [PubMed] [Google Scholar]
- Watkins W.E. & Pollitt E. (1996) Effect of removing Ascaris on the growth of Guatemalan schoolchildren. Pediatrics 97, 871–876. [PubMed] [Google Scholar]
- Watkins W.E. & Pollitt E. (1997) ‘Stupidity or worms’: do intestinal worms impair mental performance? Psychological Bulletin 121, 171–191. [DOI] [PubMed] [Google Scholar]
- West K.P. & Sommer A. (2002) Vitamin A programme in Assam probably caused hysteria. British Medical Journal 324, 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A.C. Jr (2003) Nitazoxanide: an important advance in anti‐parasitic therapy. American Journal of Tropical Medicine and Hygiene 68, 382–383. [PubMed] [Google Scholar]
- WHO (1983) Measuring Change in Nutritional Status. World Health Organization: Geneva. [Google Scholar]
- WHO (1987) Prevention and control of intestinal parasitic infections In: WHO Technical Report Series 749. World Health Organization: Geneva. [PubMed] [Google Scholar]
- WHO (1994a) Bench Aids for the Diagnosis of Intestinal Parasites. World Health Organization: Geneva. [Google Scholar]
- WHO (1994b) Report of the WHO Informal Consultation on Hookworm Infection and Anaemia in Girls and Women. World Health Orgainzation: Geneva. [Google Scholar]
- WHO (1995) WHO Model Prescribing Information. Drugs Used in Parasitic Diseases. World Health Organization: Geneva. [Google Scholar]
- WHO (2002a) Prevention and control of schistosomiasis and soil‐transmitted helminthiasis In: Technical Report Series 912. World Health Organization: Geneva. [PubMed] [Google Scholar]
- WHO (2002b) Report of the WHO Informal Consultation on the Use of Praziquantel during Pregnancy/Lactation and Albendazole/Mebendazole in Children Under 24 Months. World Health Organization: Geneva. [Google Scholar]
- WHO (2006) Preventive Chemotherapy in Human Helminthiasis. World Health Organization: Geneva. [Google Scholar]
- WHO/CDC (2005) Assessing the Iron Status of Populations. World Health Organization: Geneva. [Google Scholar]
- WHO/UNICEF (2004) How to Add Deworming to Vitamin A Distribution. World Health Organization: Geneva. [Google Scholar]
- Willett W.C., Kilama W.L. & Kihamia C.M. (1979) Ascaris and growth rates: a randomized trial of treatment. American Journal of Public Health 69, 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth J.C., Latham M.C., Hall A., Chesher A. & Crompton D.W. (1982) Worker productivity and the nutritional status of Kenyan road construction laborers. American Journal of Clinical Nutrition 36, 68–78. [DOI] [PubMed] [Google Scholar]
- World Food Programme (2006) Fact Sheet: School Feeding. World Food Programme: Rome. [Google Scholar]
- Zhou H., Ohtsuka R., He Y., Yuan L., Yamauchi T. & Sleigh A.C. (2005) Impact of parasitic infections and dietary intake on child growth in the schistosomiasis‐endemic Dongting Lake Region, China. American Journal of Tropical Medicine and Hygiene 72, 534–539. [PubMed] [Google Scholar]



