Abstract
BACKGROUND
Chemotherapy for metastatic lung or colorectal cancer can prolong life by weeks or months and may provide palliation, but it is not curative.
METHODS
We studied 1193 patients participating in the Cancer Care Outcomes Research and Surveillance (CanCORS) study (a national, prospective, observational cohort study) who were alive 4 months after diagnosis and received chemotherapy for newly diagnosed metastatic (stage IV) lung or colorectal cancer. We sought to characterize the prevalence of the expectation that chemotherapy might be curative and to identify the clinical, sociodemographic, and health-system factors associated with this expectation. Data were obtained from a patient survey by professional interviewers in addition to a comprehensive review of medical records.
RESULTS
Overall, 69% of patients with lung cancer and 81% of those with colorectal cancer did not report understanding that chemotherapy was not at all likely to cure their cancer. In multivariable logistic regression, the risk of reporting inaccurate beliefs about chemotherapy was higher among patients with colorectal cancer, as compared with those with lung cancer (odds ratio, 1.75; 95% confidence interval [CI], 1.29 to 2.37); among nonwhite and Hispanic patients, as compared with non-Hispanic white patients (odds ratio for Hispanic patients, 2.82; 95% CI, 1.51 to 5.27; odds ratio for black patients, 2.93; 95% CI, 1.80 to 4.78); and among patients who rated their communication with their physician very favorably, as compared with less favorably (odds ratio for highest third vs. lowest third, 1.90; 95% CI, 1.33 to 2.72). Educational level, functional status, and the patient’s role in decision making were not associated with such inaccurate beliefs about chemotherapy.
CONCLUSIONS
Many patients receiving chemotherapy for incurable cancers may not understand that chemotherapy is unlikely to be curative, which could compromise their ability to make informed treatment decisions that are consonant with their preferences. Physicians may be able to improve patients’ understanding, but this may come at the cost of patients’ satisfaction with them. (Funded by the National Cancer Institute and others.)
Chemotherapy remains the primary treatment approach for patients with metastatic lung or colorectal cancer. Although efficacy has improved over time, chemotherapy is not curative, and the survival benefit that has been seen in clinical trials is usually measured in weeks or months.1-3 Chemotherapy may provide some palliation, but it is also often associated with substantial treatment-related toxic effects.2-5
To make informed decisions about whether to receive chemotherapy, patients with advanced lung or colorectal cancer need a realistic understanding of its likely benefits. Previous studies have shown that patients with advanced solid tumors overestimate their life expectancy.6-9 Typically, these studies document substantial discrepancies between patients’ assessments of their life expectancy and the estimates of their physicians. Much less is known about the understanding of the effectiveness of treatment among patients with advanced cancer, a conceptually distinct topic, since unlike prognosis, there is no uncertainty about whether chemotherapy offers any prospect of cure. Several small studies, most conducted in a single clinical setting, suggest that some patients with metastatic disease believe that palliative chemotherapy could be curative,10-17 but the prevalence and determinants of this misunderstanding have not been well characterized.
Using data from the national Cancer Care Outcomes Research and Surveillance (CanCORS) study, we sought to characterize the reported expectations of patients with metastatic lung or colorectal cancer about the effectiveness of chemotherapy (and of the likelihood of cure in particular) and to identify the clinical, sociodemographic, and health-system factors associated with a reported expectation that chemotherapy might be curative.
METHODS
PATIENTS
The CanCORS study enrolled patients with newly diagnosed lung or colorectal cancer from 5 geographic regions (northern California, Los Angeles County, North Carolina, Iowa, and Alabama), 5 large health maintenance organizations, and 15 Veterans Affairs facilities.18 The cohort included approximately 10,000 patients over 20 years of age who had received the diagnosis of lung or colorectal cancer between 2003 and 2005. Potential patients were identified within weeks after the diagnosis through population-based tumor-registry rapid case ascertainment in the geographic sites and through health care systems in the integrated care sites. The characteristics of patients enrolled in CanCORS have been shown to be representative of patients identified by the Surveillance, Epidemiology, and End Results (SEER) Program for the two types of cancer.19 The study was approved by the human subjects committee at each study center.
Professional interviewers surveyed patients (or surrogates of patients who were too ill to be interviewed or had died) 4 to 7 months after diagnosis about their personal characteristics, decision making, experience of care, and outcomes.20 The interviewer used computer-assisted telephone-interview software to facilitate survey administration and response entry; patients or their surrogates did not interact with the computers in any way. Medical records of all providers who were involved in the patients’ cancer care were abstracted.
This analysis was restricted to patients with stage IV (i.e., metastatic at diagnosis) lung or colorectal cancer who opted to receive chemotherapy and who were surveyed regarding their beliefs about the effectiveness of chemotherapy. Patients who reported that they did not discuss chemotherapy with any physician or whose physician told them not to have chemotherapy were not asked this question; it was also not included in the version of the survey administered to surrogates of deceased patients or the brief version of the survey used in several data-collection sites. The members of the group are detailed in Figure S1 in the Supplementary Appendix, available with the full text of the article at NEJM.org.
DATA COLLECTION
We elicited responses from patients about the effectiveness of chemotherapy with an item adapted from the Los Angeles Women’s Health Study21: “After talking with your doctors about chemotherapy, how likely did you think it was that chemotherapy would … help you live longer, cure your cancer, or help you with problems you were having because of your cancer?” Response options were “very likely,” “somewhat likely,” “a little likely,” “not at all likely,” and “don’t know.” Refusal to respond was also recorded.
Patients were classified as having opted to receive chemotherapy if the patient or surrogate responded “yes” when asked if the patient was receiving, was scheduled to receive, or had previously received chemotherapy. Other variables that were obtained from the survey included age, sex, educational level, race or ethnic group, marital status, and household income. Patients’ reports on physician communication were calculated as described previously by transforming the sum of five items derived from the Consumer Assessment of Healthcare Providers and Systems (CAHPS)22,23 — “How often did your doctors ‖ listen carefully to you, explain things in a way you could understand, give you as much information as you wanted about your cancer treatments (including potential benefits and side effects), encourage you to ask all the cancer-related questions you had, and treat you with courtesy and respect?” — into a scale of 0 to 100 (with higher scores indicating better communication) and then categorized into tertiles. A dichotomous measure of physical functioning was based on three items from the European Quality of Life–5 Dimensions (EQ-5D),24 classifying functioning as “good” if the patient or surrogate reported no problems with mobility, no problems with self-care, and no or some problems performing usual activities and as “poor” otherwise.
Patients characterized their role in decision making about chemotherapy, as compared with the role of the physician, and their role regarding treatments in general, as compared with the role of their family. Responses were categorized as patient-controlled, shared control, or physician- or family-controlled, as described previously.25
Patients who were enrolled in the study through the Veterans Affairs and health maintenance organization (HMO) sites and those in Kaiser Permanente of Northern California or Southern California were classified as receiving their care in an integrated network. Data regarding HMO enrollment information were not available for patients at the other geographic sites because of the low penetration of HMOs in the region.
STATISTICAL ANALYSIS
We summarized reported expectations about the effectiveness of chemotherapy according to clinical, sociodemographic, and health-system factors for each type of cancer. The association between cancer type and the four-level response about treatment effectiveness (excluding “don’t know” responses) was assessed with the use of a non-parametric test for trend, an extension of the rank-sum test that incorporates a correction for ties. We used the matched-pairs signed-rank test to evaluate whether responses about cure differed from those about life extension. Factors associated with responses about likelihood of cure were analyzed with the use of multivariable logistic regression. All variables of interest were included in the multivariable model, regardless of statistical significance. The primary model examined factors that were associated with the response that chemotherapy was not at all likely to be curative and therefore classified as inaccurate any responses that chemotherapy was “very likely,” “somewhat likely,” or “a little likely” to be curative and “don’t know” or refusal to respond. Overall P values are reported for variables having three or more categories.
Results of separate models for lung and colorectal cancer were very similar (data not shown). Therefore, we included all patients in the same model with an indicator for cancer type. Sensitivity analyses were conducted to examine the effect of considering a response of “don’t know” or refusal as accurate, considering only responses of “very likely” to cure as inaccurate, and including as a covariate whether the survey was completed before or after termination of first-line chemotherapy (restricted to the 74% of patients for whom the timing of chemotherapy was documented in medical-record abstraction data).
The rate of nonresponse was less than 1% for items that were included on all versions of the baseline interview. The items on physician communication, decision-making role, and income were not included on the brief interview, and the item on physical function was not included on the surrogate interview, resulting in missing data for 7% of responses on physician communication, 6% on decision-making role, 13% on income, and 22% on physical function. Multivariable analyses were conducted on a multiply imputed data set.26,27 Imputed values were not used for descriptive data shown in Table 1, or in Table S1 and Figure S1 in the Supplementary Appendix. Statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute) and Stata, version 11.1 (StataCorp).
Table 1. Proportion of Patients with Advanced Cancers Who Responded That Chemotherapy Might Be Curative, According to the Type of Cancer.*.
| Characteristic | Lung Cancer (N = 710) |
Colorectal Cancer (N = 483) |
|---|---|---|
| percent | ||
| All patients | 69 | 81 |
| Age | ||
| 21–54 yr | 69 | 79 |
| 55–69 yr | 66 | 80 |
| 70–79 yr | 76 | 83 |
| ≥80 yr | 66 | 89 |
| Sex | ||
| Male | 71 | 80 |
| Female | 67 | 82 |
| Race or ethnic group† | ||
| White | 63 | 74 |
| Hispanic or Latino | 79 | 91 |
| Black | 82 | 91 |
| Asian or Pacific Islander | 88 | 88 |
| Other | 81 | 90 |
| Marital status | ||
| Married or living with a partner | 71 | 78 |
| Not married | 67 | 84 |
| Education | ||
| Less than high school | 75 | 86 |
| High school or some college | 68 | 82 |
| College degree or higher | 68 | 75 |
| Household income ($) | ||
| <20,000 | 66 | 82 |
| 20,000–39,999 | 67 | 81 |
| 40,000–59,999 | 73 | 81 |
| ≥60,000 | 66 | 78 |
| Integrated health care network‡ | ||
| No | 70 | 85 |
| Yes | 69 | 72 |
| Baseline interview type | ||
| Full | 70 | 80 |
| Brief | 76 | 88 |
| With surrogate for ill patient | 66 | 85 |
| Good physical function§ | ||
| No | 68 | 74 |
| Yes | 71 | 82 |
| Physician-communication score¶ | ||
| 0–79 | 61 | 72 |
| 80–99 | 69 | 77 |
| 100 | 74 | 85 |
| Patient–physician role in decision making | ||
| Patient-controlled | 67 | 80 |
| Shared control | 70 | 79 |
| Physician-controlled | 72 | 86 |
| Patient–family role in decision making | ||
| Patient-controlled | 67 | 79 |
| Shared control | 71 | 81 |
| Family-controlled | 56 | 80 |
| Survey after end of first-line chemotherapy∥ | ||
| No | 71 | 84 |
| Yes | 65 | 74 |
Listed are the responses of patients who did not respond that chemotherapy was “not at all likely” to cure their cancer.
Race or ethnic group was self-reported.
Integrated health care networks included health maintenance organizations of the Cancer Research Network, Kaiser Permanente of Northern California or Southern California, and Veterans Affairs sites.
A dichotomous measure of physical functioning was based on three items from the European Quality of Life–5 Dimensions.
Patients’ reports on physician communication were calculated by transforming the sum of five items derived from the Consumer Assessment of Healthcare Providers and Systems into a scale of 0 to 100, with higher scores indicating better communication.
This category was restricted to the 885 patients for whom data on the timing of chemotherapy were available.
RESULTS
PATIENTS
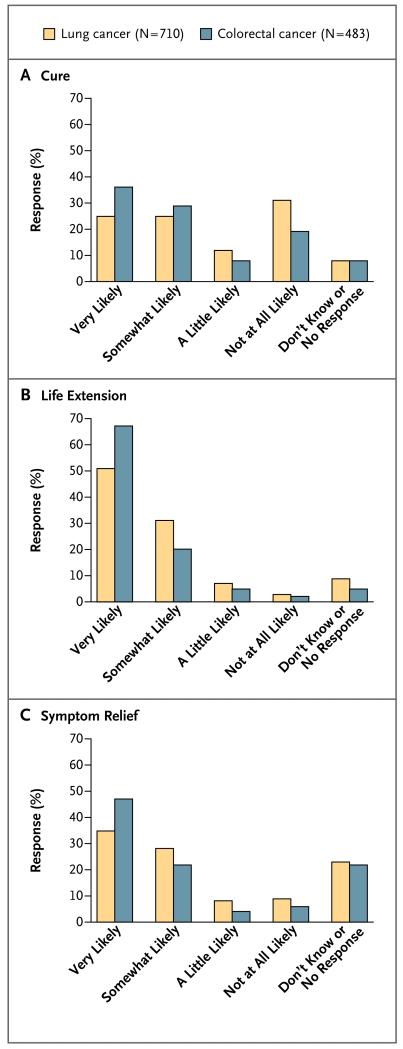
Of 1274 patients with stage IV lung or colorectal cancer who were alive at the time of the baseline survey and who discussed chemotherapy with at least one physician, 1193 (93.6%) opted to receive chemotherapy (see Table S1 in the Supplementary Appendix for patients’ characteristics). Patient-reported expectations about the effectiveness of chemotherapy for the outcomes of cure, life extension, and symptom relief are shown in Figure 1. For all outcomes, patients with colorectal cancer thought that chemotherapy was more likely to be effective than did patients with lung cancer (P<0.01 for all comparisons), and both groups believed that life extension was more likely than cure (P<0.001 for both comparisons).
Figure 1. Responses to Questions about the Likelihood That Chemotherapy Will Have an Effect, According to the Type of Effect and Diagnosis.
Shown are the responses of patients with advanced lung or colorectal cancer to questions regarding whether chemotherapy will cure their disease (Panel A), extend their life (Panel B), or provide relief of symptoms (Panel C).
EXPECTATIONS ABOUT CHEMOTHERAPY
The proportion of patients with inaccurate expectations about the likelihood that chemotherapy might cure their cancer according to patients’ characteristics is shown in Table 1. Overall, 69% of patients with lung cancer and 81% of those with colorectal cancer gave answers that were not consistent with understanding that chemotherapy was very unlikely to cure their cancer. In multivariable logistic regression, factors that were associated with a greater likelihood of this apparent misunderstanding were a diagnosis of colorectal cancer as compared with lung cancer (odds ratio, 1.75; 95% confidence interval [CI], 1.29 to 2.37; P<0.001) and nonwhite race or ethnic group as compared with white race, including Hispanic or Latino patients (odds ratio, 2.82; 95% CI, 1.51 to 5.27), black patients (odds ratio, 2.93; 95% CI, 1.80 to 4.78), and Asian or Pacific Islander patients (odds ratio, 4.32; 95% CI, 2.19 to 8.49; P<0.001 for the overall comparison) (Table 2). Patients were less likely to provide inaccurate responses if they received their care in an integrated network (odds ratio, 0.70; 95% CI, 0.52 to 0.94; P = 0.02) or if they reported lower scores for physician communication, including a score of 80 to 99 versus a score of less than 80 (odds ratio, 1.37; 95% CI, 0.93 to 2.02) and a perfect score of 100 versus a score of less than 80 (odds ratio, 1.90; 95% CI, 1.33 to 2.72; P = 0.002 for the overall comparison). None of the other factors that were examined, including education, functional status, and the patient’s role in decision making, were significantly associated with the likelihood of an inaccurate response about the curative potential of chemotherapy.
Table 2. Odds Ratios for the Association between Various Factors and an Inaccurate Response to Questions about the Likelihood of Cure with Chemotherapy.*.
| Variable | Odds Ratio (95% CI) |
P Value |
|---|---|---|
| Cancer type | <0.001 | |
| Lung | Reference | |
| Colorectal | 1.75 (1.29–2.37) | |
| Age | 0.06 | |
| 21–54 yr | Reference | |
| 55–69 yr | 1.10 (0.77–1.57) | |
| 70–79 yr | 1.68 (1.10–2.57) | |
| ≥80 yr | 1.47 (0.77–2.80) | |
| Race or ethnic group | <0.001 | |
| White | Reference | |
| Hispanic or Latino | 2.82 (1.51–5.27) | |
| Black | 2.93 (1.80–4.78) | |
| Asian or Pacific Islander | 4.32 (2.19–8.49) | |
| Other | 3.07 (1.50–6.27) | |
| Integrated health care network | 0.02 | |
| No | Reference | |
| Yes | 0.70 (0.52–0.94) | |
| Baseline interview type | 0.06 | |
| Full | Reference | |
| Brief | 2.32 (1.03–5.26) | |
| With surrogate for ill patient | 0.80 (0.54–1.19) | |
| Physician-communication score | 0.002 | |
| 0–79 | Reference | |
| 80–99 | 1.37 (0.93–2.02) | |
| 100 | 1.90 (1.33–2.72) |
Odds ratios were calculated with the use of multivariable logistic regression. An odds ratio of more than 1 represents a greater likelihood of an inaccurate belief. Listed are variables with P<0.10 in the multivariable model. Full results for all variables that were included in the multivariable model are provided in Table S2 in the Supplementary Appendix.
SENSITIVITY ANALYSES
We conducted several sensitivity analyses to assess the robustness of our findings. On the basis of the unadjusted results, we added an interaction term for cancer type to the primary model and found that the effect of care in an integrated network was stronger among patients with colorectal cancer than among those with lung cancer (odds ratio, 0.51; 95% CI, 0.28 to 0.91; P = 0.02). To assess the effect of disease progression before the survey, we restricted the analysis to patients for whom data regarding treatment duration were available. In this analysis, we found that there was no significant association between having completed the survey after the discontinuation of first-line chemotherapy and expectations regarding cure (odds ratio, 0.79; 95% CI, 0.57 to 1.10; P = 0.16).
Our primary model examined factors associated with the response that chemotherapy would not be curative. In a secondary model, we assessed factors associated with incorrect expectations, classifying “don’t know” and “refused,” as well as “not at all likely” as accurate responses. The results were very similar except that the odds ratio for care in an integrated network increased from 0.70 to 0.80 and was no longer significant (P = 0.17). Finally, we constructed a model in which only “very likely” to cure was considered an inaccurate response. The results were similar to the primary model except that there was a strong inverse association between the educational level and the likelihood of having an inaccurate expectation, including having a high-school education or some college versus having less than a high-school education (odds ratio, 0.69; 95% CI, 0.48 to 0.98) and having a college degree versus having less than a high-school education (odds ratio, 0.49; 95% CI, 0.32 to 0.77; P = 0.009 for the overall comparison).
DISCUSSION
In a national population- and health-system-based cohort of patients with recently diagnosed metastatic cancer, we found that 69% of patients with lung cancer and 81% of those with colorectal cancer who were alive 4 months after diagnosis and who had opted to receive chemotherapy provided survey responses indicating inaccurate expectations about the curative potential of chemotherapy. After accounting for the effect of other factors, including education and income, nonwhite patients remained at particularly high risk for an inaccurate response. Paradoxically, patients who reported higher scores for physician communication were also at higher risk for inaccurate expectations, whereas patients with colorectal cancer receiving their care in integrated networks appeared to be at somewhat lower risk.
The rate of inaccurate responses that we observed was substantially higher than rates reported in previous smaller studies, most of which showed that only a minority of patients believed that palliative treatment would be curative.10,11,13,15-17 However, those studies surveyed patients in tertiary care cancer centers, and most asked patients whether they believed the goal of therapy to be cure or palliation. In contrast, we surveyed a large cohort representative of patients with cancer throughout the United States and asked how likely they thought it was that chemotherapy would cure them, with probabilistic rather than dichotomous response options. Therefore, our results are likely to be more generalizable.
Should we be concerned that the majority of patients with these diseases provide responses suggesting that they do not understand that there is essentially no chance that the chemotherapy they are receiving will cure them? Chemotherapy may offer palliation and some prolongation of life, so it represents a reasonable treatment choice for some patients. However, an argument can be made that patients without a sustained understanding that chemotherapy cannot cure their cancer have not met the standard for true ongoing informed consent to their treatment.28 Furthermore, previous studies have shown that patients with advanced cancer would accept toxic treatment for even a 1% chance of cure but would be unwilling to accept the same treatment for a substantial increase in life expectancy without cure.29,30 This finding suggests that patients who do not know whether a treatment offers any possibility of cure may be compromised in their ability to make informed treatment decisions that are consonant with their preferences.29,30 Finally, and perhaps most worrisome, this misunderstanding could represent an obstacle to optimal end-of-life planning and care.
The scale of CanCORS precluded detailed patient-specific physician surveys or audiotaping of clinical encounters. Therefore, we cannot comment on what physicians told patients about the expected outcomes of chemotherapy or directly examine the roles of patients and physicians in the genesis of patients’ expectations about the likelihood of cure. Nor can we assess how many patients understood the true likelihood of cure but responded to the question in a fashion that belied their level of understanding. The fact that 20 to 30% of respondents recognized that chemotherapy was not at all likely to cure them shows that at least some patients were able to accept this reality and to acknowledge it to an interviewer. The strikingly higher rate of inaccurate responses among nonwhite patients in our cohort, a difference not explained by education or income, strongly suggests that cultural factors influence patients’ beliefs, the nature of physician–patient communication about prognosis and care, or both.
Our results also provide evidence that physicians have some ability to influence patients’ understanding. The observed association between inaccurate beliefs about the likelihood of cure and higher ratings of physician communication suggests a link between physicians’ communication behaviors and patients’ understanding of treatment benefits. This suggests that patients perceive physicians as better communicators when they convey a more optimistic view of chemotherapy. Similarly, the finding that patients, especially those with colorectal cancer, who were treated in integrated networks were somewhat more likely to understand that chemotherapy is not curative suggests that providers may be able to improve patients’ understanding if they feel it is part of their professional role.
Multiple studies have documented that oncologists usually tell patients when their disease is not curable.12,31 However, it is clear from the results of our study and other studies that disclosure alone may not lead to sustained understanding among patients. Factors that have been previously shown to contribute to patients’ lack of acceptance of medical facts include lack of trust in physicians, alternative belief systems, and use of ambiguous language by physicians.31,32 One particularly relevant cancer-specific ethnographic study showed that “collusion” between patients with cancer and their physicians played a primary role, with a quick transition by both physician and patient from discussion of prognosis to discussion of treatment options and schedule, refocusing attention and leading to false optimism.31 In other words, a focus on chemotherapy was the instrument that facilitated prognostic misunderstanding. This phenomenon may help explain our finding that patients with colorectal cancer, a more chemotherapy-responsive disease than lung cancer, were more likely to report that chemotherapy could be curative.
An extensive body of literature is designed to help physicians learn to effectively and compassionately engage patients with terminal illness in discussions of end-of-life care.33-35 Much less has been written about how to help patients recognize that treatment is not curative,36-38 and most patient-oriented public websites do not include clear information about this issue. Our results suggest that greater attention to this area is needed.
Our study has several strengths, including the large sample size, the population-based ascertainment of patients, the scope of the clinical and sociodemographic data collected, and the use of a probabilistic rather than dichotomous outcome variable. It also has several weaknesses. Because our survey was conducted several months after diagnosis and excluded surrogates of deceased patients, we cannot comment on the beliefs of patients who received chemotherapy but died soon after diagnosis. Furthermore, we conducted a single survey several months after diagnosis and therefore cannot comment on whether and how responses might change over time. To our surprise, patients who were surveyed after the end of first-line chemotherapy were no less likely to report that chemotherapy could be curative, suggesting the tenacity of this inaccurate projection even in the face of experience. Despite our efforts to minimize social-desirability bias by the use of well-trained interviewers adhering to a standard script, telephone (rather than in-person) survey administration, and reassurance to patients that their responses would not be shared with their providers,39 it is possible that patients responded with greater optimism than they actually felt and that a clinically skilled and empathic interviewer who probed more deeply into these issues could have elicited follow-up responses that showed greater awareness. Finally, although our data suggest that both patients and physicians contribute to the genesis of this misunderstanding, the CanCORS study was not designed to collect the data needed to characterize the complex psychological and communication processes that led to the responses we obtained.
In an era of greater measurement and accountability in health care, we need to recognize that oncologists who communicate honestly with their patients, a marker of a high quality of care, may be at risk for lower patient ratings. Our results suggest the need for targeted education to help all physicians learn to communicate honestly while also maintaining patients’ trust and regard. Efforts to incorporate earlier and more effective end-of-life care must address honestly and unambiguously patients’ unrealistic expectations about the outcomes of chemotherapy.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers, Dana–Farber Cancer Institute/Cancer Research Network (U01 CA093332), Harvard Medical School/Northern California Cancer Center (U01 CA093324, RAND/UCLA U01 CA093348), University of Alabama at Birmingham (U01 CA093329), University of Iowa (U01 CA093339), and University of North Carolina (U01 CA093326); and by a Department of Veterans Affairs grant to the Durham Veterans Affairs Medical Center (CRS 02-164).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glimelius B, Hoffman K, Graf W, Pahlman L, Sjoden PO. Quality of life during chemotherapy in patients with symptomatic advanced colorectal cancer. Cancer. 1994;73:556–62. doi: 10.1002/1097-0142(19940201)73:3<556::aid-cncr2820730310>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 4.Helsing M, Bergman B, Thaning L, Hero U. Quality of life and survival in patients with advanced non-small cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposide or supportive care only: a multicentre randomised phase III trial. Eur J Cancer. 1998;34:1036–44. doi: 10.1016/s0959-8049(97)10122-8. [DOI] [PubMed] [Google Scholar]
- 5.The Elderly Lung Cancer Vinorelbine Italian Study Group Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:66–72. doi: 10.1093/jnci/91.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer. 1994;2:242–4. doi: 10.1007/BF00365729. [DOI] [PubMed] [Google Scholar]
- 7.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe J, Klar N, Grier HE, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000;284:2469–75. doi: 10.1001/jama.284.19.2469. [DOI] [PubMed] [Google Scholar]
- 9.Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25:1357–62. doi: 10.1200/JCO.2006.08.3170. [DOI] [PubMed] [Google Scholar]
- 10.Eidinger RN, Schapira DV. Cancer patients’ insight into their treatment, prognosis, and unconventional therapies. Cancer. 1984;53:2736–40. doi: 10.1002/1097-0142(19840615)53:12<2736::aid-cncr2820531233>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer patients’ perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–8. doi: 10.1038/bjc.1988.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattellari M, Voigt KJ, Butow PN, Tattersall MH. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20:503–13. doi: 10.1200/JCO.2002.20.2.503. [DOI] [PubMed] [Google Scholar]
- 13.Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29:2077–84. doi: 10.1200/JCO.2010.32.0754. [DOI] [PubMed] [Google Scholar]
- 14.Gabrijel S, Grize L, Helfenstein E, et al. Receiving the diagnosis of lung cancer: patient recall of information and satisfaction with physician communication. J Clin Oncol. 2008;26:297–302. doi: 10.1200/JCO.2007.13.0609. [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29:2319–26. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 16.Burns CM, Broom DH, Smith WT, Dear K, Craft PS. Fluctuating awareness of treatment goals among patients and their caregivers: a longitudinal study of a dynamic process. Support Care Cancer. 2007;15:187–96. doi: 10.1007/s00520-006-0116-8. [DOI] [PubMed] [Google Scholar]
- 17.Chow E, Andersson L, Wong R, et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol) 2001;13:204–8. doi: 10.1053/clon.2001.9255. [DOI] [PubMed] [Google Scholar]
- 18.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–6. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Catalano P, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium relative to the Surveillance, Epidemiology and End Results (SEER) program. Med Care Appl Methods. 2012 Mar 7; doi: 10.1097/MLR.0b013e318222a711. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients’ experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–48. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen JY, Tao ML, Tisnado D, et al. Impact of physician-patient discussions on patient satisfaction. Med Care. 2008;46:1157–62. doi: 10.1097/MLR.0b013e31817924bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays RD, Shaul JA, Williams VS, et al. Psychometric properties of the CAHPS 1.0 survey measures. Med Care. 1999;37:MS22–MS231. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ayanian JZ, Zaslavsky AM, Arora NK, et al. Patients’ experiences with care for lung cancer and colorectal cancer: findings from the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2010;28:4154–61. doi: 10.1200/JCO.2009.27.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Keating NL, Beth Landrum M, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28:4364–70. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y. Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes. 2010;3:98–105. doi: 10.1161/CIRCOUTCOMES.109.875658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Zaslavsky AM, Landrum MB, Harrington DP, Catalano P. Multiple imputation in a large-scale complex survey: a practical guide. Stat Methods Med Res. 2010;19:653–70. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lidz CW, Appelbaum PS, Meisel A. Two models of implementing informed consent. Arch Intern Med. 1988;148:1385–9. [PubMed] [Google Scholar]
- 29.Slevin ML, Stubbs L, Plant HJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ. 1990;300:1458–60. doi: 10.1136/bmj.300.6737.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQuellon RP, Muss HB, Hoffman SL, Russell G, Craven B, Yellen SB. Patient preferences for treatment of metastatic breast cancer: a study of women with early-stage breast cancer. J Clin Oncol. 1995;13:858–68. doi: 10.1200/JCO.1995.13.4.858. [DOI] [PubMed] [Google Scholar]
- 31.The AM, Hak T, Koeter G, van Der Wal G. Collusion in doctor-patient communication about imminent death: an ethnographic study. BMJ. 2000;321:1376–81. doi: 10.1136/bmj.321.7273.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Char SJ, Evans LR, Malvar GL, White DB. A randomized trial of two methods to disclose prognosis to surrogate decision makers in intensive care units. Am J Respir Crit Care Med. 2010;182:905–9. doi: 10.1164/rccm.201002-0262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–60. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 34.Smith TJ, Hillner BE. Explaining marginal benefits to patients, when “marginal” means additional but not necessarily small. Clin Cancer Res. 2010;16:5981–6. doi: 10.1158/1078-0432.CCR-10-1278. [DOI] [PubMed] [Google Scholar]
- 35.Robinson K, Sutton S, von Gunten CF, et al. Assessment of the Education for Physicians on End-of-Life Care (EPEC) Project. J Palliat Med. 2004;7:637–45. doi: 10.1089/jpm.2004.7.637. [DOI] [PubMed] [Google Scholar]
- 36.von Gunten CF, Lupu D. Development of a medical subspecialty in palliative medicine: progress report. J Palliat Med. 2004;7:209–19. doi: 10.1089/109662104773709332. [DOI] [PubMed] [Google Scholar]
- 37.Von Roenn JH, von Gunten CF. Setting goals to maintain hope. J Clin Oncol. 2003;21:570–4. doi: 10.1200/JCO.2003.10.161. [DOI] [PubMed] [Google Scholar]
- 38.Smith TJ, Dow LA, Virago EA, Khatcheressian J, Matsuyama R, Lyckholm LJ. A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer. J Support Oncol. 2011;9:79–86. doi: 10.1016/j.suponc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourangeau R, Rips LJ, Rasinski K. The psychology of survey response. Cambridge University Press; Cambridge, United Kingdom: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.