Abstract
Muscle atrophy (cachexia) is a muscle wasting syndrome associated with several pathological conditions in humans such as congestive heart failure, diabetes, AIDS, cancer and renal failure, and the presence of cachexia worsens outcome. Many of the conditions associated with cachexia are accompanied by stimulation of the reninangiotensin system and elevation in angiotensin II (ang II) levels. Ang II infusion induces skeletal muscle atrophy in rodents and mechanisms include increased expression of the E3 ligases atrogin-1/MuRF-1, an elevated rate of ubiquitin-proteasome mediated proteolysis and increased reactive oxygen species (ROS) levels, closely mimicking conditions of human cachexia. Ang II-induced oxidative stress contributes to muscle atrophy in a mouse model. Nicotinamide adenine dinucleotide phosphate oxidase- and mitochondria-derived ROS contribute to ang II-induced oxidative stress. Specific targeting of ROS and nicotinamide adenine dinucleotide phosphate oxidase/mitochondria cross-talk could be a beneficial, novel therapy to treat cachexia.
Keywords: Angiotensin II, Muscle atrophy, Oxidative stress
Muscle atrophy is defined as a decrease in muscle mass. Muscle atrophy or muscle wasting can be acute if it results from disuse and this type of atrophy is reversible after exercise. Chronic atrophy is characterized by a progressive and sustained decline in muscle mass. Sarcopenia, an age-related muscle weight loss, represents this type of chronic atrophy. In humans, some authors have suggested that cachexia is considered to be present when >7% muscle loss is evident1; however, no specific threshold has been established yet for animal models of muscle atrophy. Chronic muscle atrophy resulting from disease rather than disuse is often defined as cachexia. Cachexia can result from muscle pathology such as muscular dystrophy, myotonia congenita (muscle damage) or systemic disease, such as poliomyelitis, diabetes, cancer, renal failure, AIDS, sepsis or congestive heart failure (CHF).2,3 The presence of cachexia significantly worsens outcome and increases mortality in patients with chronic disease. In this review, we will focus on mechanisms of muscle atrophy described in humans and in animal models, with special emphasis on the role of oxidative stress in angiotensin II (ang II)-induced muscle wasting.
MECHANISMS OF SKELETAL MUSCLE ATROPHY
The regulation of skeletal muscle mass involves a dynamic process of anabolic and catabolic reactions, which ultimately results in maintenance of a specific level of muscle proteins. The reduction in muscle proteins is a consequence of an anabolic/catabolic imbalance, namely reduced protein synthesis and increased protein breakdown/proteolysis. Muscle atrophy is characterized by the activation of distinct pathways, in particular the ATP-dependent ubiquitin-proteasome proteolysis pathway. In genetic screens aimed at identifying markers of muscle atrophy, 2 genes in particular, atrogin-1/MAFbx and MuRF1, were dramatically up-regulated before the onset of muscle loss in multiple experimental models.4 – 6 Both these genes encode proteins that are E3 ubiquitin ligases. E3 ligases are enzymes responsible for the substrate specificity of ubiquitin conjugation, and, therefore, these molecules are essential for proteasome-mediated protein degradation. Because genetic deletion of either atrogin-1/MAFbx or MuRF1 resulted in a partial protection against muscle wasting,4 the molecular regulation of these 2 proteins is of great significance with regard to understanding mechanisms of muscle atrophy.
Proteasome system activation in atrophied skeletal muscle can also be mediated by increased expression of proteasome subunits7 and caspase-3-dependent cleavage of specific protea-some subunits.8 Four 20S proteasome catalytic core subunits α1 (Psma1), α5 (Psma5), β3 (Psmb3) and β4 (Psmb4) were up-regulated in multiple types of skeletal muscle atrophy,9 and levels of the β1 subunit (Psmb1) were up-regulated in skeletal muscle of patients with cancer with weight loss.10 Wang et al8 have recently demonstrated that caspase-3 increased protea-some activity through specific cleavage of Rpt2 and Rpt6 subunits. They found that in mice with a muscle wasting condition, chronic kidney disease, there was cleavage of subunits Rpt2 and Rpt6, which was associated with increased proteasome activity. Taken together, these findings establish a critical role of the ubiquitin-proteasome system in muscle atrophy.
An important question related to muscle wasting is whether atrophy (resulting from increased protein catabolism) and the converse process of muscle hypertrophy (resulting from increased protein anabolism) occurs through 2 independent processes in parallel or if these mechanisms are coregulated. Insulin-like growth factor I (IGF-1) and insulin have been shown to promote net protein accumulation (ie, hypertrophy) of mature myotubes and adult muscle fibers. Low levels of insulin and IGF-1, together with elevated levels of glucocorticoids, induce the loss of muscle protein in diabetes,11 and insulin resistance is a characteristic feature of many systemic diseases that seems to contribute to muscle wasting. In skeletal muscle, the binding of IGF-1 or insulin to its cognitive receptor activates 2 major signaling pathways: the Ras-Raf-MEK-ERK pathway and the PI3K/AKT pathway. The Ras-Raf-MEK-ERK pathway affects fiber type composition without significant effects on muscle fiber size,12 whereas activation of the PI3K/AKT pathway induces muscle hypertrophy by regulation of GSK and mTOR kinases.13 Results from different groups suggest that decreased activity of the IGF-1/PI3K/AKT signaling pathway can lead to muscle atrophy.13–15 Glucocorticoid-treated myotubes have an increase in protein degradation rate and atrogin-1 expression, and these effects were suppressed by IGF-1.16 PI3K inhibitors induced protein degradation and up-regulated expression of atrogin-1 in these myotubes. These experiments suggest that the IGF-1/PI3K/AKT pathway down-regulates expression of atrogin-1 and suppresses degradation of proteins, including myofibrillar proteins. A major downstream target of the PI3K/AKT pathway is the Forkhead box O (FoxO) class of transcription factors. FoxO1 is an atrophy-related gene that is activated in many types of muscle atrophy.9 Sandri et al16 demonstrated that IGF-1 acts through AKT and FoxO1 to suppress atrogin-1 transcription and that the mTOR/S6K, GSK and NFκB pathways are not important in regulating atrogin-1 expression.
ANGIOTENSIN II-INDUCED MUSCLE ATROPHY
Ang II, the main effector molecule of the renin-angiotensin system (RAS), has multiple physiological effects including the maintenance of sodium and water balance through a variety of effects on the central nervous system, the adrenal gland, the vasculature and the kidney. In addition to regulating blood pressure and salt/water balance, the RAS plays an important role in the pathogenesis of all stages of cardiovascular disease, ranging from early endothelial dysfunction to target-organ damage, CHF, renal or cerebrovascular disease.18 Patients with advanced cardiovascular or renovascular disease, such as CHF or end-stage renal disease, often have cachexia, which independently worsens outcome.19 These patients often have increased ang II levels,20,21 suggesting that ang II could play an important role in cachexia. Indeed, several literature reports have suggested that angiotensin-converting enzyme inhibitors may improve weight loss in CHF22 and that ang II may play a role in cancer cachexia.23 However, angiotensin-converting enzyme inhibitors and genetic interference with RAS components prevented weight gain in rodent models of obesity24–26 by reducing food intake, perhaps by increasing brain ang II levels.
Brink et al27 found, in 1996, that ang II infusion in the rat caused a significant loss in body weight, which was largely accounted for by a reduction in food intake and was pressor independent. Because the anorexigenic effect of ang II (decrease in food intake) clearly contributed to ang II-induced muscle wasting, they performed experiments with an additional control group, pair-fed animals which received an amount of food identical to ang II-treated rats. Based on pair-feeding experiments, they demonstrated that weight loss and muscle wasting induced by ang II were not only due to reduced food intake but also due to a catabolic effect. The effect of ang II to produce muscle atrophy was paralleled by marked changes in levels of circulating IGF-1 and its binding proteins (IGFBP-2 and IGFBP-3).27 This group subsequently demonstrated that ang II-induced muscle wasting occurs by increased muscle protein degradation through activation of the ubiquitin-proteasome pathway and this effect was associated with increased apoptosis.27 Ang II disruption of IGF-1 signaling in muscle played a critical role in the atrophic effect of ang II,28 so that muscle-specific overexpression of IGF-1 prevented ang II-induced activation of the proteasome system and muscle wasting.29 Using electroporation of cDNA constructs into skeletal muscle, Yoshida et al30 recently demonstrated that the ability of IGF-1 to prevent ang II-induced wasting was mediated by an AKT- and a FoxO-1-dependent signaling pathway that resulted in inhibition of atrogin-1 but not of MuRF-1 expression. Of note, ang II can also disrupt insulin signaling in muscle, potentially contributing to its wasting effect.31
Ang II receptors (AT1 and AT2 class receptors) mediate the majority of ang II effects in vitro and in vivo32; however, an unresolved issue in the biology of the RAS system is to what extent angiotensin receptors are involved in the direct effects of ang II on skeletal muscle. Some studies report that ang II acts directly on myotubes to increase protein degradation,23,33 but we and others34 have found little evidence of significant expression of ang II receptors on mature skeletal muscle cells. Zhang et al34 demonstrated that ang II induced hepatic IL-6 and serum amyloid A (SAA1) production in the mouse and these mediators acted synergistically to disrupt insulin/IGF-1 signaling and promote skeletal muscle proteolysis. Ang II stimulated the suppressor of cytokine signaling (SOCS3), which led to marked skeletal muscle atrophy and these responses were suppressed in IL-6-deficient mice or potentiated by a SAA1-overexpressing adenovirus,34 suggesting that ang II effects on skeletal muscle were mediated by SAA1/IL-6-dependent signaling pathways. Glucocorticoids have been shown to be required for stimulation of the ubiquitin-proteasome pathway in diabetes, fasting and metabolic acidosis,35–37 and we have shown that ang II infusion increases glucocorticoid levels28 and that RU486, a glucocorticoid receptor antagonist, significantly blocked the ang II-induced weight loss.29 These data suggest that the ability of ang II to increase glucocorticoids play an important role in ang II-induced skeletal muscle atrophy.
Ang II stimulates the release of the adrenal cortical hormone aldosterone38 and increases circulating levels of catecholamines39 and the vasoconstrictor endothelin-1.40 The potential contribution of these molecules to ang II-induced skeletal muscle wasting increases the complexity of mechanisms involved in muscle loss.
ROLE OF OXIDATIVE STRESS
Oxidative stress generally refers to the increased cellular production of reactive oxygen species (ROS), molecules or ions formed by the incomplete 1-electron reduction of oxygen. These reactive oxygen intermediates include singlet oxygen, superoxides, peroxides, hydroxyl radical and hypochlorous acid. ROS are involved in the regulation of normal cell signaling and cell growth, proliferation and expansion of the extra-cellular matrix.41 ROS can be produced by almost any cell types, including vascular endothelial, smooth muscle cells, blood mononuclear cells, cardiomyocytes and skeletal muscle cells. ROS are generated by activation of xanthine oxidase, lipoxygenases, nitric oxide synthase, the mitochondrial respiratory chain and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymatic complex. The relative contribution of these oxidases to ROS generation depends on cell-specific differences in oxidase levels and in cellular organelles involved in the generation of ROS (such as mitochondria). Increased ROS can, in turn, directly inflict tissue injury or can contribute to the production of additional ROS, for instance, by converting nitric oxide into peroxynitrite, which is also injurious to tissues. Oxidative stress is a common hallmark of several pathological conditions including CHF, atherosclerosis, diabetes and cancer.42
Kondo43 has shown that increased ROS contributed to disuse muscle atrophy. This work revealed that immobilization of skeletal muscles was associated with oxidative injury and muscle atrophy was retarded by the delivery of exogenous antioxidants. The lipid-soluble antioxidant vitamin E reduced immobilization-induced muscle atrophy by 20% in these experiments43 and the ability of vitamin E to diminish disuse muscle atrophy was further confirmed by another group.44 Prevention of oxidant stress through the administration of the antioxidant cysteine effectively suppressed protein ubiquitination and myosin heavy chain fragmentation in the gastrocnemius muscle after hind limb suspension in rats,45 suggesting that oxidative stress contributes to disuse muscle atrophy by regulation of proteolysis. Powers et al46 have postulated that there are 3 mechanisms by which skeletal muscle oxidative stress contributes to the increased rate of muscle proteolysis and atrophy: (1) oxidative stress leads to calcium overload and activation of calcium-activated proteases (eg, calpain); (2) oxidative stress activates caspase-3 and through this mechanism stimulates the 20S proteasome system; (3) oxidative stress up-regulates expression of atrogin-1 and MuRF-1 in muscle and these E3 ligases consequently activate the proteasome system. In addition, p38MAP kinase was recently identified as a novel link between oxidative stress and increased expression of both ubiquitin-proteasome and autophagy-related genes in atrophied skeletal muscle.47
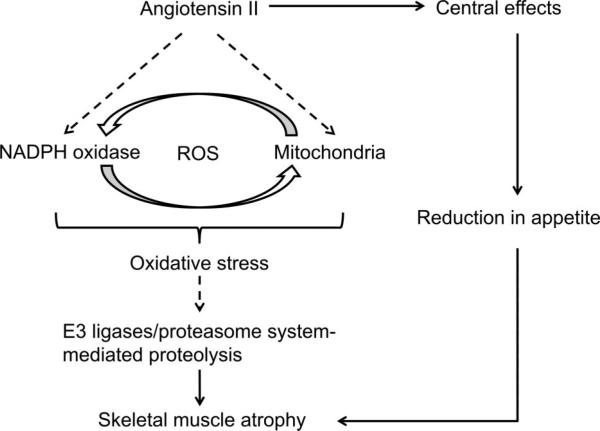
Several reports suggest that 2 oxidant systems, namely NADPH oxidase and mitochondria, are major sources of ROS in atrophied skeletal muscles (Figure 1). Whitehead et al48 found the up-regulated expression of NADPH oxidase subunits and increased levels of NADPH oxidase-derived ROS in skeletal muscle of dystrophic mdx mice. Pharmacological inhibition of NADPH oxidase in these experiments reduced the intracellular Ca2+ rise in mdx single fibers after stretched contractions and also attenuated the loss of muscle force. These results suggest that NADPH oxidase is a main ROS source in dystrophic muscle and that its enhanced activity stimulates stretch-induced Ca2+ entry, an important mechanism for muscle damage and functional impairment. Muller et al49 reported a marked increase in mitochondrial ROS generation in 3 conditions associated with muscle atrophy: in aging, in mice lacking the antioxidant enzyme CuZn-SOD (Sod1–/– mice) and in the neurodegenerative disease, amyotrophic lateral sclerosis. ROS generation in muscle mitochondria was 3-fold higher in aged mice versus young controls, and it was associated with a 30% loss in gastrocnemius muscle mass. In Sod1–/– mice, muscle mitochondrial ROS production was increased more than 2-fold along with a >50% loss in muscle mass and amyotrophic lateral sclerosis G93A mutant mice show a 75% loss of muscle mass during disease progression and up to 12-fold higher mitochondrial ROS levels. These data show that mitochondrial ROS production is strongly correlated with the extent of muscle atrophy in these models.
FIGURE 1.
Hypothetic mechanism of Angiotensin II (ang II)-induced muscle wasting involving oxidative stress. Ang II increases both nicotinamide adenine dinucleotide phosphate (NADPH) oxidase- and mitochondrial-derived reactive oxygen species (ROS) in skeletal muscle and reduces appetite through central effects. NADPH oxidase/mitochondria crosstalk mechanism amplifies ROS and activates proteasome system-mediated muscle protein degradation. Loss of appetite and proteasome activation contributes to ang II-induced skeletal muscle atrophy. Dashed lines depict hypothetical pathways.
Increased ROS levels have been detected in skeletal muscle from ang II-treated rodents. ROS levels were elevated in muscles from ang II-infused rats in parallel with up-regulation of gp91phox, a NADPH oxidase subunit.50 Wei et al51 demonstrated that ang II markedly enhanced NADPH oxidase activity and consequent ROS generation in L6 myotubes and these effects were blocked by the AT1 receptor blocker, losartan, and also by the NADPH oxidase inhibitor, apocynin. It is of note that Dai et al52 recently reported that ang II stimulates mitochondrial ROS and induces mitochondrial dysfunction in cardiac myocytes. Overexpression of the mitochondria-targeted antioxidant enzyme catalase reduces mitochondria-derived ROS and blocked ang II-induced cardiac hypertrophy. Taken together, these data demonstrate that ang II increases ROS in different cell types (including skeletal muscle cells); however, it is unclear whether the increase in ROS mediates skeletal muscle atrophy. A few important findings came recently from our own laboratory. Semprun-Prieto et al53 have demonstrated that ang II-induced muscle wasting correlated with 2.5-fold increase in gastrocnemius muscle superoxide levels and the increase in both ROS and muscle atrophy was blocked by the antioxidant N-acetylcysteine. Interestingly, skeletal muscle-specific IGF-1 overexpression also decreased ang II-induced oxidative stress in skeletal muscle and, potentially, by using this mechanism, IGF-1 prevented muscle atrophy.54 These data are consistent with the critical importance of ROS for ang II-induced muscle wasting. Recently, we found that ang II-induced ROS were blocked by treatment with apocynin, suggesting NADPH oxidase involvement. Moreover, we found that mitochondria-derived superoxides were increased in skeletal muscle of ang II-infused animals.55 These results indicate involvement of dual oxidases (NADPH oxidase and the mitochondrial system) in ang II-induced muscle atrophy. Our data also raised the question about the role of mitochondria/NADPH oxidase cross-talk in ang II-induced oxidative stress and muscle wasting. Doughan et al56 described a potential mechanism of ang II-induced mitochondrial dysfunction, which involves NADPH oxidase-derived ROS, as recently summarized by Daiber.57 Briefly, ang-II activates NADPH oxidase and induces subsequent ROS formation. NADPH oxidase-dependent ROS activate mitochondrial ATP-sensitive potassium channels leading to depolarization of the mitochondrial membrane. Decreased mitochondrial membrane potential mediates the increase in mitochondrial ROS (mostly hydrogen peroxide) and activates protein kinase C, triggering the cycle of subsequent NADPH oxidase activation. Thus, ang II-stimulated NADPH oxidase serves as the primary source of ROS and mitochondria act as an amplifier for increased oxidative stress. This mechanism was described for endothelial cells56; however, supportive findings were also reported for vascular smooth muscle cells58 and for rat cardiac ischemic reperfusion injury.59 A detailed study of NADPH oxidase/mitochondria cross-talk in atrophied skeletal muscle is a promising direction for future investigation.
CONCLUSIONS
Muscle atrophy (cachexia) is a muscle wasting syndrome associated with several pathological conditions in humans such as CHF, diabetes, AIDS, cancer and renal failure, and the presence of cachexia worsens outcome. Many of the conditions associated with cachexia are accompanied by stimulation of the RAS and elevation in ang II levels. Ang II infusion induces skeletal muscle atrophy in rodents and mechanisms include increased expression of E3 ligases atrogin-1/MuRF-1, an elevated rate of ubiquitin-proteasome mediated proteolysis and increased ROS levels, closely mimicking conditions of human cachexia. Ang II-induced oxidative stress contributes to muscle atrophy in a mouse model. NADPH oxidase- and mitochondria-derived ROS contribute to ang II-induced oxidative stress. Specific targeting of ROS and NADPH oxidase/mitochondria cross-talk could be a beneficial, novel therapy to treat cachexia.
Acknowledgments
This study was supported by the NIH grants RO1 HL070241 and RO1 HL080682.
Footnotes
The authors declare no conflict of interest.
Presented as a State-of-the-Art Lecture at the Southern Society for Clinical Investigation Cardiovascular Club Session, February 18, 2011, New Orleans, LA.
REFERENCES
- 1.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–6. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 3.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 4.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 5.Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–5. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson EJ, Giresi PG, Koncarevic A, et al. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551(Pt 1):33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price SR. Increased transcription of ubiquitin-proteasome system components: molecular responses associated with muscle atrophy. Int J Biochem Cell Biol. 2003;35:617–28. doi: 10.1016/s1357-2725(02)00385-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang XH, Zhang L, Mitch WE, et al. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J Biol Chem. 2010;285:21249–57. doi: 10.1074/jbc.M109.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 10.Khal J, Hine AV, Fearon KC, et al. Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int J Biochem Cell Biol. 2005;37:2196–206. doi: 10.1016/j.biocel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Mitch WE, Bailey JL, Wang X, et al. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276(5 Pt 1):C1132–8. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- 12.Murgia M, Serrano AL, Calabria E, et al. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol. 2000;2:142–7. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- 13.Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 14.Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 15.Pallafacchina G, Calabria E, Serrano AL, et al. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA. 2002;99:9213–8. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacheck JM, Ohtsuka A, McLary SC, et al. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287:E591–601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 17.Sandri M, Sandri C, Gilbert A, et al. FoxO transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner C, Poss J, Bohm M. Optimal antagonism of the Renin-Angiotensin-aldosterone system: do we need dual or triple therapy? Drugs. 2010;70:1215–30. doi: 10.2165/11537910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–7. doi: 10.1097/MCO.0b013e328300ecc1. [DOI] [PubMed] [Google Scholar]
- 20.Masson S, Latini R, Bevilacqua M, et al. Within-patient variability of hormone and cytokine concentrations in heart failure. Pharmacol Res. 1998;37:213–7. doi: 10.1006/phrs.1998.0288. [DOI] [PubMed] [Google Scholar]
- 21.Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–7. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Negassa A, Coats AJ, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–83. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005;93:425–34. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kloet AD, Krause EG, Kim DH, et al. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009;150:4114–23. doi: 10.1210/en.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100:525–34. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Kloet AD, Woods SC. Molecular neuroendocrine targets for obesity therapy. Curr Opin Endocrinol Diabetes Obes. 2010;17:441–5. doi: 10.1097/MED.0b013e32833c3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–16. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brink M, Price SR, Chrast J, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142:1489–96. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- 29.Song YH, Li Y, Du J, et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–8. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Semprun-Prieto L, Sukhanov S, et al. IGF-1 prevents ANG II-induced skeletal muscle atrophy via AKT- and FoxO-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol. 2010;298:H1565–70. doi: 10.1152/ajpheart.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond-Stanic MK, Henriksen EJ. Direct inhibition by angiotensin II of insulin-dependent glucose transport activity in mammalian skeletal muscle involves a ROS-dependent mechanism. Arch Physiol Biochem. 2010;116:88–95. doi: 10.3109/13813451003758703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011;20:84–8. doi: 10.1097/MNH.0b013e3283414d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell ST, Sanders PM, Tisdale MJ. Angiotensin II directly inhibits protein synthesis in murine myotubes. Cancer Lett. 2006;231:290–4. doi: 10.1016/j.canlet.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Du J, Hu Z, et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–12. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986;77:614–21. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing SS, Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol. 1993;264(4 Pt 1):E668–76. doi: 10.1152/ajpendo.1993.264.4.E668. [DOI] [PubMed] [Google Scholar]
- 37.May RC, Bailey JL, Mitch WE, et al. Glucocorticoids and acidosis stimulate protein and amino acid catabolism in vivo. Kidney Int. 1996;49:679–83. doi: 10.1038/ki.1996.96. [DOI] [PubMed] [Google Scholar]
- 38.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins TA, Allen AM, Chai SY, et al. Interactions of angiotensin II with central catecholamines. Clin Exp Hypertens. 1995;17:267–80. doi: 10.3109/10641969509087070. [DOI] [PubMed] [Google Scholar]
- 40.Rossi GP, Sacchetto A, Cesari M, et al. Interactions between endothelin-1 and the renin-angiotensin-aldosterone system. Cardiovasc Res. 1999;43:300–7. doi: 10.1016/s0008-6363(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 41.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 42.Kaneto H, Katakami N, Matsuhisa M, et al. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo H. Handbook of oxidants and antioxidants in exercise. 1st ed. Elsevier; Amsterdam; New York: 2000. pp. 631–53. [Google Scholar]
- 44.Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–60. doi: 10.1055/s-2007-972612. [DOI] [PubMed] [Google Scholar]
- 45.Ikemoto M, Nikawa T, Kano M, et al. Cysteine supplementation prevents unweighting-induced ubiquitination in association with redox regulation in rat skeletal muscle. Biol Chem. 2002;383:715–21. doi: 10.1515/BC.2002.074. [DOI] [PubMed] [Google Scholar]
- 46.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–44. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 47.McClung JM, Judge AR, Powers SK, et al. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298:C542–9. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitehead NP, Yeung EW, Froehner SC, et al. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One. 2010;5:e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller FL, Song W, Jang YC, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1159–68. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Swanson SA, Ye J, et al. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension. 2006;48:637–43. doi: 10.1161/01.HYP.0000240347.51386.ea. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y, Sowers JR, Nistala R, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–46. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 52.Dai DF, Johnson SC, Villarin JJ, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and G{alpha}q overexpression-induced heart failure. Circ Res. 2011;108:837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semprun-Prieto L, Sukhanov S, Yoshida T, et al. Angiotensin II induced muscle wasting is redox dependent: differential requirement of p47 phox in the anorexigenic and catabolic effects of angiotensin II [Abstract 3578]. Circulation. 2009;120(18):S829. MeetingAbstracts. [Google Scholar]
- 54.Semprun-Prieto L, Sukhanov S, Yoshida T, et al. Skeletal musclespecific overexpression of insulin-like growth factor 1 decreases ocidative stress and prevents angiotensin II-induced skeletal muscle wasting: novel potential therapy to treat cachexia in congestive heart failure.. J Investig Med; AFMR Southern Regional Meeting; New Orleans, LA. February 12–14, 2009; 2009. p. 320. [Abstract] [Google Scholar]
- 55.Sukhanov S, Semprun-Prieto L, Yoshida T, et al. Dual oxidase involvement in angiotensin II-induced oxidative stress in wasted skeletal muscle.. J Investig Med; Southern Regional Meeting; February 25–27, 2010; 2010. p. 443. [Abstract] [Google Scholar]
- 56.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 57.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Wosniak J, Jr, Santos CX, Kowaltowski AJ, et al. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–78. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 59.Kimura S, Zhang GX, Nishiyama A, et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardio-protection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–6. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]