Abstract
Objective
To study whether pain location is related to lesion location in women with chronic pelvic pain and biopsy-proven endometriosis.
Methods
A secondary analysis was performed to compare self-reported pain location with recorded laparoscopy findings for location and characteristics of all visible lesions. All lesions were excised. Endometriosis was diagnosed using histopathology criteria. The pelvic area was divided into three anterior and two posterior regions. Lesion depth, number of lesions or endometriomas, and disease burden (defined as sum of lesion sizes, or single versus multiple lesions) were determined for each region. Data were analyzed using t-tests, Fisher’s exact tests, and logistic regression modeling, with p-values corrected for multiple comparisons using the step-down Bonferroni method.
Results
Women with endometriosis (n=96) had a lower body mass index (BMI), were more likely to be white, had more prior surgery, and had more frequent menstrual pain and incapacitation than chronic pain patients without endometriosis (n=37). Overall, few patients had deeply infiltrating lesions (n=38). Dysuria was associated with superficial bladder peritoneal lesions. Other lesions or endometriomas were not associated with pain in the same anatomic locations. Lesion depth, disease burden, and number of lesions or endometriomas were not associated with pain.
Conclusion
In this group of women with biopsy-proven endometriosis, few had deeply infiltrating lesions or endometriomas. Dysuria and midline anterior pain were the only symptoms associated with the location of superficial endometriosis lesions. The lack of relationship between pain and superficial lesion location raises questions about how these lesions relate to pain.
Clinical Trial Registration
ClinicalTrials.gov, www.clinicaltrials.gov, NCT00001848.
Introduction
Endometriosis is a benign gynecologic condition associated with pain and infertility. Women with chronic pelvic pain are frequently diagnosed with endometriosis or pelvic adhesions. In subjects with endometriosis, lysis of adhesions and removal of deeply infiltrating endometriosis (DIE) lesions, endometriomas, and peritoneal lesions has been the surgical approach to treatment, with the assumption that removing these lesions may alleviate pain.
Improvement in pain is more likely with surgical treatment of lesions than with diagnostic laparoscopy alone. However, not all women experience pain relief. About 20% of study participants had no improvement after surgical excision of endometriosis in a randomized controlled trial (1), while 30% experienced less pain after diagnostic laparoscopy without biopsy. A meta-analysis (2) of three randomized blinded trials showed only a 30–40% short-term improvement in pain with surgical removal of lesions as compared with observational laparoscopy; this effect decreased with time, and the rate of reoperation approached 50%.
Some studies report that specific surgical findings or lesion number are associated with pelvic pain symptoms. In a retrospective study of 225 women with pelvic pain symptoms and DIE, Fauconnier et al. (3) noted that adhesions and DIE nodules in various anatomic locations were associated with dysmenorrhea, dyspareunia, lower urinary tract symptoms, or GI symptoms. Similarly, a retrospective study by Vercellini et al. (4) analyzed the anatomic characteristics of lesions and the type and severity of pain symptoms for 1054 women undergoing surgery for various indications, finding a strong association between dyspareunia and posterior cul-de-sac lesions.
Perper et al. (5) reported that the number of endometriosis implants correlated positively with pain severity. Others have shown that lesion location correlates with symptoms, such that deep vaginal lesions (6) and uterosacral lesions (7) are associated with dyspareunia. The correlation between pain location and lesion location has been more difficult to establish. Perper et al. (5) reported no correlation between pain and lesion location, and others (6, 7) have shown that pain and American Society of Reproductive Medicine (ASRM) stage of endometriosis did not correlate.
The objective of this study of women with chronic pelvic pain and biopsy-proven endometriosis was to estimate whether pain location was related to lesion location and other lesion characteristics, including lesion depth, number, and color. All women in this study had laparoscopic removal of all visible lesions suspicious for endometriosis. In addition, urinary, gastrointestinal, and coital symptoms were analyzed for their relation to the presence of DIE lesions in specific anatomic locations.
Materials and Methods
Women with chronic pelvic pain and symptoms suggestive of endometriosis underwent laparoscopy from January 1999 to December 2004 at the National Institutes of Health (NIH) Clinical Center or Georgetown University after Institutional Review Board approval (8). Those with biopsy-proven endometriosis then participated in a clinical trial to assess the effectiveness of raloxifene in delaying the return of pain. Entry criteria included women aged 18 to 45 years with regular menses and at least three months of chronic pelvic pain. Study participants had a body mass index (BMI) less than 40 kg/m2 and were in excellent health, except for the use of antidepressants, medications for migraines and headaches, and allergy medications. All subjects agreed to postpone pregnancy during the study and were abstinent, used barrier contraception, or were sterilized.
Exclusion criteria included leuprolide or surgical treatment of endometriosis within the past six months, hormonal contraception (or selective estrogen receptor modulators, progestins, estrogens, steroids, or ovulation induction) within the past three months, hysterectomy or bilateral salpingo-oophorectomy, pregnancy, or lactation. After giving informed consent, study participants underwent a standardized diagnostic evaluation for causes of pelvic pain, which included a history and physical examination and referral to other specialists for evaluation, based on clinical signs and symptoms. Women with manic depressive illness, or untreated major depression, and those with chronic pelvic pain resulting only from infectious, gastrointestinal (including irritable bowel syndrome), musculoskeletal (including fibromyalgia), neurologic, or psychiatric causes were also excluded. Additionally, women with abnormal screening results for myofascial diseases associated with pain (including antinuclear antibodies, creatinine kinase, thyroid function, and rheumatoid arthritis tests) were referred for evaluation (8).
We compared baseline pain symptoms with surgical findings in this clinical trial, in which all lesions were excised by the same surgical team and then reviewed by a pathologist. Thus, this study is a secondary analysis of the baseline survey and laparoscopy data from the raloxifene study above (8). Information was acquired on pain type, location, duration and severity on a standardized preoperative questionnaire. History of endometriosis and other pelvic symptoms such as dyspareunia, dysuria, dyschezia, menstrual and nonmenstrual pain were recorded at baseline before surgery. The severity of menstrual, nonmenstrual, and coital pain was also recorded on an 11-point visual analog scale (VAS) [0 (no pain) to 10 (worst pain imaginable)]. Dysuria was defined as any pain with urination in the absence of a urinary tract infection. Dyschezia was defined as painful defecation or rectal pain, and gastrointestinal symptoms included dyschezia, constipation, diarrhea, and rectal bleeding.
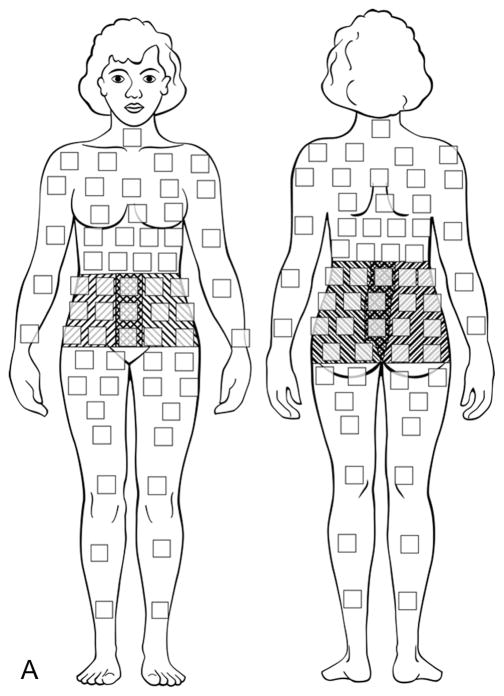
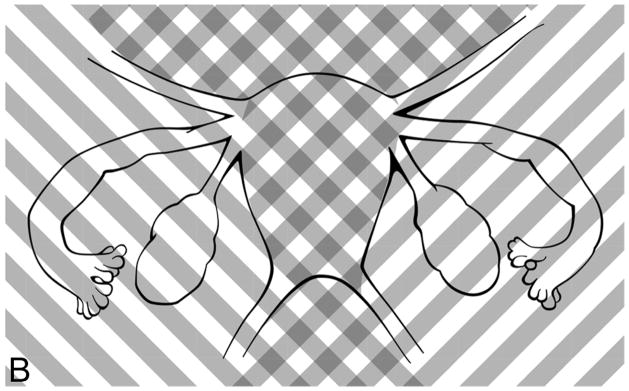
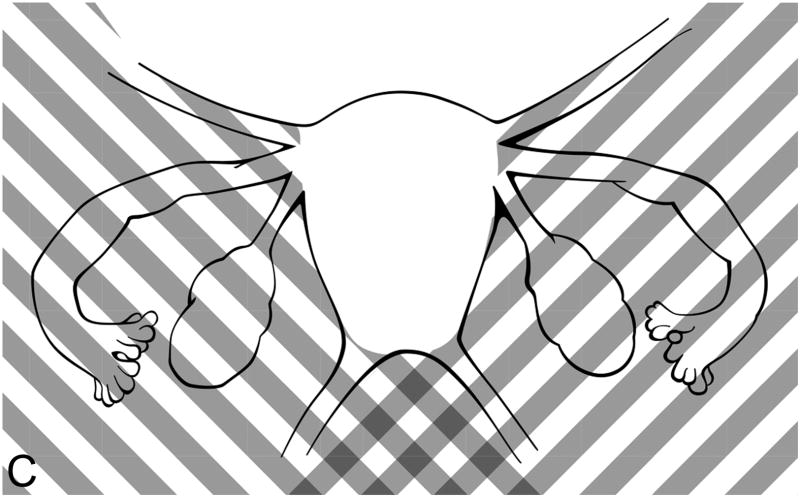
Study participants depicted all areas of abdominal and pelvic pain on a computerized diagram (Figure 1a), on which they were instructed to select the small boxes which corresponded to their areas of pain (9). Surgical findings were recorded, each suspicious endometriosis lesion was excised, and the resulting specimens were sent for histopathologic evaluation of endometriosis (Figures 1b and 1c) (10). In order to compare the self-reported anatomic locations with findings from surgery, pelvic pain and anatomic locations were then grouped into five regions: posterior left and right, and anterior left, middle, and right (Figures 1a–1c). As the research team noted that women with chronic pelvic pain always chose an anterior abdomino-pelvic region to show their “pelvic” pain and sometimes also chose a region on their back, lesions were categorized by the left or right side of the body, recognizing that pain might be referred to either the front or the back. Thus, anterior right included all structures on the right side: the right sidewall, right ovarian fossa, right fallopian tube, right ovary, right uterosacral ligament, and appendix. Anterior middle corresponded to the bladder, uterus, and cul-de-sac. The anterior left included all structures on the left side of the pelvis, including the left sidewall, left ovarian fossa, left fallopian tube, left ovary, left uterosacral ligament, and sigmoid colon. The posterior areas were similar to the anterior ones, but did not include a separate middle region over the spine. Thus, the right posterior region included the right sidewall, right ovarian fossa, right uterosacral ligament, appendix, and cul-de-sac extending to (but not including) the left uterosacral. The posterior left also began at the cul-de-sac (without the right uterosacral) and included the left sidewall, left ovarian fossa, left uterosacral ligament, and sigmoid colon.
Figure 1.
Figure 1a. Computerized diagram of pain location regions for patient surveys. Upward right shading, anterior right and posterior right; cross-hatches, anterior middle; upward left shading, anterior and posterior left. Note that the posterior middle was included with both the posterior left and posterior right regions. Modified from: Management of endometriosis in the presence of pelvic pain. The American Fertility Society. Fertil Steril 1993 Dec;60(6):952–5, Copyright 1993, with permission from Elsevier.
“Figure 1b. Diagram of anterior pain regions for surgically-scored lesions. Upward right shading, anterior right; cross-hatches, anterior middle; upward left shading, anterior left.
Figure 1c. Diagram of posterior pain regions for surgically-scored lesions. Upward right shading, posterior right; upward left shading, posterior left. Note that the posterior middle was included with both the posterior left and posterior right regions. Figures 1b and 1c modified from: Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997 May;67(5):817–21, Copyright 1997, with permission from Elsevier.
The goal of laparoscopy was to physically remove any pigmented or non-pigmented implants suspicious for endometriosis, so the diagnosis of endometriosis could be assessed by pathology; these lesions included deeply infiltrating lesions, peritoneal lesions and endometriomas(8). Using a standardized surgical approach, one investigator (P.S.) operated at nearly all (131 of 133) laparoscopic surgeries to assure consistency; lesions were excised, not ablated, using a neodymium:yttrium argon garnet contact laser (Surgical Laser Technologies, The Oaks, PA). The location and characteristics (including size, depth, and color) of each lesion were systematically recorded at surgery on the diagram in Figure 1b with information regarding lesion details, which was then provided to pathology.
Women were diagnosed with endometriosis if endometrial glands and stroma were identified in at least one specimen on histopathology, and only biopsy-proven lesions were included in this analysis. Lesion diameter was calculated using the average of two width measurements, with depth calculated as a single measurement. Deep infiltrating endometriosis (DIE) was defined as any lesion ≥5 mm in depth. Endometriomas were pseudocyst lesions within the ovary and confirmed by histology. In each of the five regions defined above, lesions were categorized as biopsy-proven lesions or endometriomas. Lesion characteristics of maximum depth, total number of lesions and endometriomas, and disease burden were also tabulated. Disease burden was defined in various ways, including the sum of lesion sizes, single versus multiple lesions per region, and whether both pain and lesions were diffuse (defined as at least three out of five regions) or localized. For this analysis, red, pink, and clear lesions were categorized as “red,” black or blue or brown lesions were considered “black,” and white and yellow implants were noted as “white.” Any combination of these three categories was considered “mixed.”
Comparisons were done using chi-square, Fisher’s exact, t-tests, Wilcoxon rank-sum, and ANOVA tests, as appropriate. Univariable logistic regression modeling was used to assess predictive associations. Group comparisons depended on the type and normality of data, with a p-value ≤ 0.05 considered statistically significant. Corrections for multiple comparisons were carried out by the step-down Bonferroni method(11). Data were analyzed using SAS v 9.1 (SAS Institute, Inc, Carey, NC).
Results
Of 133 study participants undergoing laparoscopy, 96 women had biopsy-confirmed endometriosis. Two patients with a “frozen pelvis” were categorized as “no endometriosis” because no biopsied lesions showed endometriosis. Those with (n=96) and without (n=37) biopsy-proven endometriosis were similar with respect to age, gravidity, and parity (Table 1). Subjects with endometriosis had a lower BMI (25.0 ±5.3 vs 29.0 ±6.5, p=0.001) and were more likely to be white (p<0.03). Women with histology-confirmed endometriosis had more prior gynecologic surgery (75% vs 54%). More than two-thirds of women with endometriosis had minimal or mild disease. Almost half of both groups had adhesions, and 20% of study participants with endometriosis had at least one endometrioma. Women with endometriosis had more menstrual pain (98.9% vs 87.9%, p<0.02) and incapacitation due to menstrual pain (22.8% vs 12.1%, p=0.01). There were no significant differences in the frequency or severity of nonmenstrual pelvic and abdominal pain, which was reported by almost all participants in both groups. Approximately one-third of both groups of women had pain persisting throughout the menstrual cycle. Results on coital pain were restricted to the 51.2% of women reporting sexual activity, with over two-thirds of women in both groups reporting discomfort with sexual activity or intercourse as painful to the point of interruption (Table 1).
Table 1.
Baseline Characteristics of Study Cohort with Chronic Pelvic Pain: Demographic Information and Characteristics of Patients With and Without Biopsy-Proven Endometriosis
| Demographics/Characteristics | Histology-confirmed endometriosis (n=96) | No endometriosis (n=37) | Total (n=133) | P-value |
|---|---|---|---|---|
| Age (mean ± SD) | 31.7 ± 7.5 | 30.8 ± 6.5 | 31.5 ± 7.2 | 0.525 |
| BMI† (mean ± SD) | 25.0 ± 5.3 | 29.0 ± 6.5 | 26.1 ± 5.9 | 0.001 |
| Gravidity (mean ± SD) | 1.0 ± 1.7 | 1.3 ± 1.8 | 1.1 ± 1.7 | 0.214 |
| Parity (mean ± SD) | 0.5 ± 1.0 | 0.8 ± 1.4 | 0.6 ± 1.1 | 0.399 |
| Race* (n, %) | 0.026 | |||
| -White | 77 (80.2) | 23 (62.2) | 100 (75.2) | |
| -Black | 15 (15.6) | 9 (24.3) | 24 (18.1) | |
| -Hispanic | 2 (2.1) | 3 (8.1) | 5 (3.8) | |
| -Asian | 2 (2.1) | 0 | 2 (1.5) | |
| -Other | 0 | 2 (5.4) | 2 (1.5) | |
| Pelvic pain duration, years (mean ± SD) | 8.8 ± 5.5 | 10.9 ± 7.5 | 10.3 ± 7.1 | 0.215 |
| Prior endometriosis surgeries* (n, %) | 72 (75.0) | 20 (54.1) | 92 (69.2) | 0.023 |
| -Laparoscopy only | 63 (87.5) | 14 (70.0) | 77 (83.7) | |
| -Laparotomy only | 1 (1.4) | 2 (2.2) | 3 (3.3) | |
| -Both | 8 (11.1) | 4 (20.0) | 12 (13.0) | |
|
| ||||
|
Characteristics at study surgery
| ||||
| AFS stage of endometriosis† (n, %) | <0.001 | |||
| -0 | 0 | 22 (59.5) | 23 (17.3) | |
| -I | 29 (30.2) | 10 (27.0) | 38 (25.6) | |
| -II | 40 (41.7) | 3 (8.1) | 43 (32.3) | |
| -III | 17 (17.7) | 0 | 17 (12.8) | |
| -IV | 10 (10.4) | 2 (5.4)‡ | 12 (9.0) | |
| Adhesions (n, %) | 47 (49.0) | 19 (51.4) | 66 (49.6) | 0.848 |
| Endometriomas (n, %)† | 18 (18.8) | 0 | 18 (13.5) | 0.003 |
| Menstrual Pain* (n, %) | 91 (98.9) | 29 (87.9) | 120 (96.0) | 0.017 |
| -Severity (mean +/− SD)§ | 6.7 ± 2.5 | 5.8 ± 3.1 | 6.5 ± 2.7 | 0.163 |
| -Some lost work efficiency (n, %) | 39 (42.4) | 9 (27.3) | 48 (38.4) | 0.012 |
| -Occasional work loss* (n, %) | 31 (33.7) | 16 (48.5) | 47 (37.6) | |
| -Incapacitated* (n, %) | 21 (22.8) | 4 (12.1) | 25 (20.0) | |
| Non-menstrual Pain (n, %) | 88 (95.7) | 31 (93.9) | 119 (95.2) | 0.654 |
| -Severity (mean +/− SD)§ | 5.2 ± 2.5 | 5.4 ± 2.7 | 5.3 ± 2.6 | 0.676 |
| -Occasional discomfort (n, %) | 36 (39.1) | 8 (24.2) | 44 (35.2) | |
| -Discomfort most of menstrual cycle (n, %) | 26 (28.3) | 12 (36.4) | 38 (30.4) | 0.434 |
| -Pain persists throughout cycle (n, %) | 26 (28.3) | 11 (33.3) | 37 (29.6) | |
| Coital Pain (n, %) | 32 (68.1) | 12 (70.6) | 44 (68.8) | 1.000 |
| -Severity (mean +/− SD)§ | 3.8 ± 3.4 | 4.3 ± 3.5 | 3.9 ± 3.4 | 0.605 |
| -Tolerated discomfort (n, %) | 16 (38.1) | 4 (26.7) | 20 (35.1) | |
| -Intercourse interrupted due to pain (n, %) | 15 (35.7) | 7 (46.7) | 22 (38.6) | 0.651 |
| -Intercourse avoided due to pain (n, %) | 1 (2.4) | 1 (6.7) | 2 (3.5) | |
p<0.05,
p<0.01
by visual inspection only. Two women with stage IV disease (frozen pelvis) were not diagnosed with endometriosis because tissue could not be removed for histological confirmation.
based on an 11-point visual analog scale (VAS) between 0 (no pain) and 10 (worst pain imaginable)
For the 96 women with chronic pelvic pain and biopsy-proven endometriosis, Table 2 estimates the possible association of lesions or endometriomas with pain in matching anatomical regions for these subjects. The presence of lesions or endometriomas was not associated with pain in the same region for any of the five regions, after correcting for multiple comparisons. Lesion characteristics, such as depth, number, color, and disease burden were similarly not predictive of pain in each of the regions. In a sub-analysis to determine whether pain localized to the same or contralateral side as the lesion in subjects with only a single endometriosis lesion (n=27), we found that all (27 of 27) women had ipsilateral pain while a majority (22 of 27) had contralateral pain as well; no participants had pain only contralateral to their solitary lesion.
Table 2.
Predictive Association of Presence and Characteristics of Endometriosis Lesions with Pain, by Anatomic Region
| Anterior Right | Anterior Middle | Anterior Left | Posterior Right | Posterior Left | |
|---|---|---|---|---|---|
| Presence | |||||
| Lesion(s) or Endometrioma(s) | 3.7 (1.1, 11.8) | 2.4 (1.1, 5.3) | 1.2 (0.5, 2.7) | 1.5 (0.6, 3.4) | 0.9 (0.4, 2.1) |
| Characteristics | |||||
| Lesion Depth or Endometrioma diameter | 1.3 (0.9, 1.9) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.1) | 0.9 (0.9, 1.1) | 0.9 (0.9, 1.0) |
| Disease burden (sum of lesion sizes) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) |
| Number of lesions and endometriomas | 1.5 (0.8, 2.6) | 1.3 (0.9, 1.9) | 1.1 (0.7, 1.6) | 1.0 (0.8, 1.3) | 1.0 (0.7, 1.3) |
Data are presented as odds ratios (OR) and their 95% confidence intervals.
All p-values were not statistically significant.
All p-values were corrected for multiple comparisons using the Stepdown Bonferroni method.
As noted in Table 3, dysuria was associated with bladder peritoneal lesions (OR 3.5 [95% CI 1.2–10.6], p<0.03). Dyschezia or dyspareunia, however, were not associated with lesions on the colon or sigmoid, cul-de-sac, or uterosacrals, regardless of lesion depth. In contrast to previous reports, an association between either cul-de-sac or uterosacral lesions and dyspareunia was not observed.
Table 3.
Predictive Association of Endometriosis Lesions with Dysuria, Dyschezia, and Dyspareunia
| Dysuria | Dyschezia | Dyspareunia | |
|---|---|---|---|
| Bladder lesions | *3.5 (1.2, 10.6) | ‡ | ‡ |
| Colon/Sigmoid lesions | ‡ | 1.6 (0.2, 11.8) | ‡ |
| Cul de sac lesions | ‡ | 1.3 (0.6, 2.8) | 0.8 (0.4, 1.7) |
| DIE† lesions in the cul-de-sac | ‡ | 1.8 (0.6, 5.1) | 0.4 (0.1, 1.5) |
| Cul de sac or uterosacral lesions | ‡ | 1.6 (0.8, 3.5) | 0.8 (0.4, 1.7) |
| DIE† lesions in cul de sac or uterosacral ligaments | ‡ | 1.2 (0.5, 3.1) | 0.6 (0.2, 1.6) |
p<0.03, statistically significant association based on logistic regression modeling
All p-values were corrected for multiple comparisons using the Stepdown Bonferroni method.
DIE = deep infiltrating endometriosis (>= 5mm)
not applicable
Discussion
Pain and symptom survey results were compared with laparoscopy findings in a secondary analysis of a study investigating the use of raloxifene after surgery, to explore associations between pain location and biopsy-proven endometriosis lesion location. Neither lesions nor lesion characteristics were predictive of pain in the same anatomic locations. While dysuria predicted peritoneal lesions on the bladder, we found no association between dyspareunia and cul-de-sac or uterosacral lesions.
The strengths of our study include the systematic collection of data about pain and other symptoms, the collection of surgical data by a skilled observer in consultation with the surgical team in the operating room, and histopathologic confirmation of endometriosis for each lesion in every subject. Using the same surgical team limits interobserver variability, but may limit the generalizability of findings. Other studies acquired data on patient symptoms and surgical findings from medical records which may be incomplete. Having a dedicated research team to comprehensively catalog lesions and pain symptoms is important to evaluate relationships between pain location and lesion location or other lesion characteristics.
In two similar studies of women with DIE lesions, severe dyspareunia and painful defecation during menses was associated with posterior DIE (12), and those with rectal or vaginal DIE lesions reported increased severity of dysmenorrhea (13). In the retrospective study of 225 women by Fauconnier et al. (3), severe dysmenorrhea was associated with adhesions in the pouch of Douglas, dyspareunia with uterosacral ligament DIE, lower urinary tract symptoms with bladder DIE, and gastrointestinal symptoms with bowel or vaginal DIE. Thus, DIE nodules may trigger pain and their removal may be therapeutic (3, 14).
Similar findings were reported by Vercellini et al. (4) in a retrospective study of 1054 women undergoing surgery for pain, pelvic mass, or infertility, a strong association between posterior cul-de-sac lesions and pain at intercourse was noted. Unlike Vercellini et al, our study has too few subjects with DIE in the cul de sac to assess this association. In the Vercellini study, Endometriosis stage was weakly associated with nonmenstrual pain, pelvic symptom severity, and dysmenorrhea severity, which was of questionable significance in their large population.
Laparoscopy under local anesthesia, or conscious pain mapping, may add information to visual inspection of the pelvis in women with chronic pelvic pain (15). While probing pelvic structures and visible pathology in a standardized fashion, subjects rate any pain on a 0–10 VAS scale. Focal tenderness may be treated with local anesthetic, re-examined, and re-scored for pain. With this technique, some subjects with pain localized to the gallbladder (16) and appendix (17) improved after cholecystectomy or appendectomy, respectively.
In studies of conscious pain mapping and endometriosis (18, 19), most women localized pain to their endometriotic lesions. However, many participants (19), as well as 10 of 11 subjects in a similar study (20), demonstrated generalized visceral hypersensitivity, in which all areas of the pelvis and bowel were sensitive, and pain was not completely blocked with local anesthesia.
In a prospective study, Demco et al. (21) reported that a majority of participants had correct left-right pelvic orientation for provoked pain location during laparoscopy under conscious sedation. However, half of the cohort perceived pain on the opposite side or referred to another location. Referred pain and right-left disorientation may be altered by thalamic misinterpretation of input from the spinal cord, or by viscero-visceral or viscera-somatic convergence within the nervous system (21, 22). Similarly, our inability to relate lesion location with pain location may be influenced by generalized hypersensitivity. Indeed, our sub-analysis of participants with only a single endometriosis lesion noted most women had generalized pain, experiencing both ipsilateral and contralateral pain.
As opposed to other studies (4, 5, 7) which combined pain and infertility cohorts, our study population was comprised exclusively of women with chronic pain. We systematically evaluated and excluded women with other causes of chronic pelvic pain, such as irritable bowel syndrome or fibromyalgia. Despite our efforts to exclude these other causes, we did not find any association between the location of pain and the location of superficial lesions. Whether other causes of pelvic pain are sought is not consistently addressed in other studies of subjects with endometriosis. Thus, endometriosis may not be the cause of pain. Further, there is tremendous heterogeneity in women with chronic pain such that the severity, associated symptoms, and initiating conditions vary from one individual to another.
A particular strength of our study was the histologic confirmation of laparoscopic diagnoses of endometriosis, occurring in two-thirds of biopsies in this cohort (23, 24). Visual diagnosis of endometriosis by laparoscopy has poor sensitivity and specificity. A “positive” finding on laparoscopy is incorrect in up to half of cases (25), while biopsy of normal-appearing peritoneum has shown endometriosis in 6% of women with no visible lesions (26). Visual diagnosis may be challenging because of the differing color, size, and morphology of peritoneal lesions (23, 24). Only 75.6% and 72.9% of women in studies by Fauconnier et al. (3) and Vercellini et al. (4), respectively, had biopsy-confirmed endometriosis. The possible miscategorization of up to one-fourth of subjects as having endometriosis, when they may not actually have the disease, may hamper inferring any association or lack of association between lesions and pain.
The inherent limitations of our study include possible selection bias and study design limitations. Most subjects were self-referred and only had minimal or mild disease at surgery. The study goal was to treat women with complete excision of all lesions at laparoscopy. Since bowel resections were not performed in this study; our findings are not generalizable to women with extensive disease involving the bladder, ureters, and bowel. All subjects underwent excision of all suspicious lesions, but some lesions may have been missed even with careful inspection. Secondly, there may be inaccuracies in translating how women ascribe their pain to a pelvic region, as well as in how lesion characteristics and location were recorded. We attempted to overcome these potential inaccuracies by defining anatomic regions broadly.
Third, the analysis was restricted to biopsy-proven endometriosis; whether some lesions were innervated while others were not was not studied here. Fourth, this analysis only estimated the relationship between pain and biopsy-proven endometriosis; the relationship between pain and adhesions or other non-endometriotic lesions was not considered. While adhesions were recorded, we were unable to infer whether adhesions related to endometriosis or prior surgery caused pain. Finally, since this study investigated secondary outcomes of a larger clinical trial, it may not be adequately powered (aggregate power yielded 65% using a two-sided alpha of 0.05) for the observed results, thus inflating the type 2 error. However, type 1 error was minimized by correcting p-values for multiple comparisons. This study had insufficient power to assess the relationship between deeply infiltrating lesions and pelvic pain.
The inconsistency of the relation between the presence or severity of pain with endometriosis lesion location, extent of disease, and lesion characteristics also emphasizes that pelvic pain is a symptom which may also arise in the setting of other regional pain syndromes such as migraines (27, 28), irritable bowel syndrome, painful bladder syndrome, and fibromyalgia (22, 29). These diseases each engage the nervous system in one of several mechanisms (22, 27). Most of our subjects, including those with a single endometriosis lesion, experienced diffuse pain which may reflect central nervous system sensitization. The fact that patients experience a region of pain in the setting of endometriosis may, in fact, be the reason why lesion location is not correlated with pain symptoms.
Sensitization is a form of neural plasticity that includes allodynia, hyperalgesia, and a lowered pain pressure threshold affecting multiple adjacent spinal cord segments. Bajaj et al. (30) has reported central sensitization and generalized hypersensitivity in women diagnosed with endometriosis, compared to healthy volunteers. “Generalized visceral hypersensitivity” described in conscious pain mapping studies also suggests sensitization. Possible mechanisms suggested by Stratton and Berkley (22) include incomplete resection of lesions, which may activate nociceptor activity after surgery, as well as damage to peripheral axons of nociceptor (pain receptor) afferents during surgery, which in turn initiates visceral pain which can persist or is easily reinitiated after surgery.
Overall, we did not find a statistically significant relationship between pain location and the location of superficial endometriosis lesions. This raises questions about how these lesions relate to pain. Referred pain, inconsistent innervation of lesions, and viscero-viscero and viscero-somatic convergence may result in pain that does not map to endometriosis lesions and endometriomas. The interaction between lesions, local nerve fibers, and the central nervous system may be more significant than the existence of endometriosis lesions in any particular anatomic location. Improving treatment of women with endometriosis-related pain will likely require a better understanding of these pain mechanisms to determine whether they are initiated and perpetuated by inflammatory or other neuropathic mechanisms, and to determine the role hormones may play in such mechanisms. This may lead to insights into which patients may benefit from surgery, and may aid in tailoring hormonal or other treatments.
Acknowledgments
The authors thank Patricia Moyer for creating an electronic version of the pain diagram for patients to complete, and Jeremy Swan and Nichole Jonas for their graphics expertise in modifying the figures for this manuscript.
Supported by an endometriosis research travel award to Society for Gynecologic Investigation for Albert Hsu from the Endometriosis Association, International Headquarters, Milwaukee Wisconsin; the Intramural Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (NCT00001848); and the NIH Clinical Center.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the Society for Gynecologic Investigation, March 24–27 2010, Orlando, Florida.
References
- 1.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004 Oct;82(4):878–84. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Vigano P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Human reproduction update. 2009 Mar–Apr;15(2):177–88. doi: 10.1093/humupd/dmn062. [DOI] [PubMed] [Google Scholar]
- 3.Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Breart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002 Oct;78(4):719–26. doi: 10.1016/s0015-0282(02)03331-9. [DOI] [PubMed] [Google Scholar]
- 4.Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007 Jan;22(1):266–71. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 5.Perper MM, Nezhat F, Goldstein H, Nezhat CH, Nezhat C. Dysmenorrhea is related to the number of implants in endometriosis patients. Fertil Steril. 1995 Mar;63(3):500–3. doi: 10.1016/s0015-0282(16)57416-0. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996 Feb;65(2):299–304. [PubMed] [Google Scholar]
- 7.Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999 Nov;6(4):429–34. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- 8.Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, et al. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstetrics and gynecology. 2008 Jan;111(1):88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Management of endometriosis in the presence of pelvic pain. The American Fertility Society. Fertil Steril. 1993 Dec;60(6):952–5. [PubMed] [Google Scholar]
- 10.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997 May;67(5):817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 11.Holm S. A simple sequentially rejective bonferroni test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 12.Chapron C, Barakat H, Fritel X, Dubuisson JB, Breart G, Fauconnier A. Presurgical diagnosis of posterior deep infiltrating endometriosis based on a standardized questionnaire. Hum Reprod. 2005 Feb;20(2):507–13. doi: 10.1093/humrep/deh627. [DOI] [PubMed] [Google Scholar]
- 13.Chapron C, Fauconnier A, Dubuisson JB, Barakat H, Vieira M, Breart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod. 2003 Apr;18(4):760–6. doi: 10.1093/humrep/deg152. [DOI] [PubMed] [Google Scholar]
- 14.Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Human reproduction update. 2005 Nov–Dec;11(6):595–606. doi: 10.1093/humupd/dmi029. [DOI] [PubMed] [Google Scholar]
- 15.Palter SF. Microlaparoscopy under local anesthesia and conscious pain mapping for the diagnosis and management of pelvic pain. Current opinion in obstetrics & gynecology. 1999 Aug;11(4):387–93. doi: 10.1097/00001703-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tytherleigh MG, Fell R, Gordon A. Diagnostic conscious pain mapping using laparoscopy under local anaesthetic and sedation in general surgical patients. Surgeon. 2004 Jun;2(3):157–60. doi: 10.1016/s1479-666x(04)80077-5. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OD, Jr, Val-Gallas JM, Rizk B. Appendectomy under local anaesthesia following conscious pain mapping with microlaparoscopy. Hum Reprod. 1998 Mar;13(3):588–90. doi: 10.1093/humrep/13.3.588. [DOI] [PubMed] [Google Scholar]
- 18.Demco L. Mapping the source and character of pain due to endometriosis by patient-assisted laparoscopy. J Am Assoc Gynecol Laparosc. 1998 Aug;5(3):241–5. doi: 10.1016/s1074-3804(98)80026-1. [DOI] [PubMed] [Google Scholar]
- 19.Howard FM, El-Minawi AM, Sanchez RA. Conscious pain mapping by laparoscopy in women with chronic pelvic pain. Obstetrics and gynecology. 2000 Dec;96(6):934–9. doi: 10.1016/s0029-7844(00)01056-5. [DOI] [PubMed] [Google Scholar]
- 20.Palter SF, Olive DL. Office microlaparoscopy under local anesthesia for chronic pelvic pain. J Am Assoc Gynecol Laparosc. 1996 May;3(3):359–64. doi: 10.1016/s1074-3804(96)80064-8. [DOI] [PubMed] [Google Scholar]
- 21.Demco LA. Pain referral patterns in the pelvis. J Am Assoc Gynecol Laparosc. 2000 May;7(2):181–3. doi: 10.1016/s1074-3804(00)80037-7. [DOI] [PubMed] [Google Scholar]
- 22.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human reproduction update. 2011 May–Jun;17(3):327–46. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stegmann BJ, Funk MJ, Sinaii N, Hartmann KE, Segars J, Nieman LK, et al. A logistic model for the prediction of endometriosis. Fertil Steril. 2009 Jan;91(1):51–5. doi: 10.1016/j.fertnstert.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stegmann BJ, Sinaii N, Liu S, Segars J, Merino M, Nieman LK, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008 Jun;89(6):1632–6. doi: 10.1016/j.fertnstert.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. Bjog. 2004 Nov;111(11):1204–12. doi: 10.1111/j.1471-0528.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 26.Balasch J, Creus M, Fabregues F, Carmona F, Ordi J, Martinez-Roman S, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod. 1996 Feb;11(2):387–91. doi: 10.1093/humrep/11.2.387. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero S, Pretta S, Bertoldi S, Anserini P, Remorgida V, Del Sette M, et al. Increased frequency of migraine among women with endometriosis. Hum Reprod. 2004 Dec;19(12):2927–32. doi: 10.1093/humrep/deh537. [DOI] [PubMed] [Google Scholar]
- 28.Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011 Mar 1;95(3):895–9. doi: 10.1016/j.fertnstert.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002 Oct;17(10):2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003 Sep;4(7):372–80. doi: 10.1016/s1526-5900(03)00720-x. [DOI] [PubMed] [Google Scholar]