Abstract
Parkinson’s disease (PD) has been associated with mild cognitive impairment (PDMCI) and with dementia (PDD). Using radial distance mapping, we studied the 3D structural and volumetric differences between the hippocampi, caudates, and lateral ventricles in 20 cognitively normal elderly (NC), 12 cognitively normal PD (PDND), 8 PDMCI, and 15 PDD subjects and examined the associations between these structures and Unified Parkinson’s Disease Rating Scale (UPDRS) Part III:motor subscale and Mini-Mental State Examination (MMSE) performance. There were no hippocampal differences between the groups. 3D caudate statistical maps demonstrated significant left medial and lateral and right medial atrophy in the PDD vs. NC, and right medial and lateral caudate atrophy in PDD vs. PDND. PDMCI showed trend-level significant left lateral caudate atrophy vs. NC. Both left and right ventricles were significantly larger in PDD relative to the NC and PDND with posterior (body/occipital horn) predominance. The magnitude of regionally significant between-group differences in radial distance ranged between 20–30% for caudate and 5–20% for ventricles. UPDRS Part III:motor subscale score correlated with ventricular enlargement. MMSE showed significant correlation with expansion of the posterior lateral ventricles and trend-level significant correlation with caudate head atrophy. Cognitive decline in PD is associated with anterior caudate atrophy and ventricular enlargement.
Keywords: Parkinson disease dementia (PDD), mild cognitive impairment (MCI), hippocampal atrophy, caudate atrophy, ventricular enlargement
BACKGROUND
Cognitive decline is common in patients with Parkinson’s disease (PD). Several postmortem studies have examined the substrate underlying dementia in PD (PDD).1 However, the anatomical changes underlying the initial stages of cognitive impairment in PD are not well understood.
Imaging studies have demonstrated cortical atrophy associated with dementia and in mild cognitive impairment in PD (PDMCI).2,3 However, inconsistent findings have been reported for the hippocampus and the caudate nucleus. In non-demented PD (PDND), two studies using the region of interest (ROI) technique4,5 and two using a visual scale reported hippocampal atrophy,6,7 whereas one ROI technique8 and several voxel-based morphometry (VBM) technique9–11 studies did not. Similarly, inconsistent reports of caudate atrophy in PD have been published.12,13 One study estimating the width of the frontal horns of the lateral ventricles reported frontal horn enlargement in PD.14
We applied several advanced neuroimaging techniques to a dataset consisting of PDND, PDMCI, PDD, and cognitively normal elderly (NC). We hypothesized that cognitive decline would be associated with hippocampal and caudate atrophy and ventricular enlargement. We applied the radial distance technique to create 3D statistical and correlation maps of the structures of interest that allow us to visualize regionally specific differences in PDND, PD-MCI, and PDD.
SUBJECTS AND METHODS
Subjects
The study was approved by the Regional Committee for Medical Research Ethics at the University of Bergen, Norway. The patient sample has been described in detail previously.3 Briefly, the cohort consisted of 12 PDND, 8 PDMCI, and 15 PDD patients and 20 NC from the same region. Diagnosis of clinically definite PD required at least two of the three cardinal symptoms: tremor, rigidity, and akinesia, and at least a moderate response to dopaminergic agents based on clinical judgment (for more details see Ref. 15). Diagnosis of dementia was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) as described previously.3,16 MCI diagnosis was rendered when individual cognitive scores on at least one of three cognitive domains fell 1.5 SD below the mean of a NC group matched on the basis of age-, sex- and education to our PDND subjects (see Ref. 17 for more details). PD motor symptom severity was assessed with the Unified PD Rating Scale (UPDRS) Part III:motor subscale18 during the “on”-phase in subjects with motor fluctuations.
Image Preprocessing
All subjects were scanned on a 1.5T Phillips Gyro-scan NT intra release 8.1 (Phillips Medical Systems, Best, The Netherlands) at Stavanger University Hospital in Norway. 3D T1-weighted spoiled gradient recalled echo images were obtained with time to repetition (TR) 12.4 ms, time to echo (TE) 4.2 ms, acquisition matrix 256 × 192, and slice thickness 1.6 mm. T1-weighted, T2-weighted, and fluid-attenuated inversion recovery sequences were also obtained and examined for significant white or gray matter pathology precluding enrollment in this study. The images were subjected to intensity19 and spatial normalization to the International Consortium for Brain Mapping ICBM53 brain atlas as previously described.20 The mean interval between clinical evaluation and MRI imaging was 37 days (range 0–93 days).
Hippocampal Segmentation
The hippocampal formations were manually segmented on gapless coronal slices by one experienced rater (AEG, intra-rater reliability Cronbach’s Alpha = 0.98) blinded to subjects’ age, gender and diagnosis following a detailed well-established protocol.21 The hippocampal traces included the hippocampus proper, dentate gyrus, and subiculum.
Caudate Segmentation
The caudate nuclei were segmented with a new automated machine-learning approach. The technique, based on a statistical method called adaptive boosting or AdaBoost, has been described in detail in Morra et al., 2008.22 Briefly, this machine-learning algorithm applies a pattern recognition approach for image segmentation. It tests iteratively various combinations of imaging features such as image intensity, x, y, and z positions, image curvature, image gradients, and the outputs of several other local image filters of various sizes, and determines the combination of features that most reliably segments a small training dataset from the study cohort (in this case 20 subjects) that have been segmented manually by an expert (AEG, intra-rater reliability Cronbach’s Alpha = 0.948). The automated traces are then compared to the manual traces for accuracy. The final AdaBoost algorithm was applied to the full study cohort. For more details on AdaBoost and its reliability please see Morra et al., 2008.22
Caudate tracing commenced in the coronal plane, but all three views (coronal, axial, and sagital) were used for anatomic guidance. The first trace was drawn on the most frontal coronal slice, where caudate gray matter was clearly visible on the inferolateral side of the superior horn of the lateral ventricle. The last trace was drawn on the most posterior slice, where the body/tail of the caudate was clearly visible in the superolateral side of the body of the lateral ventricle. As the body of the caudate thinned out and transitioned into the caudate tail, the differentiation of the caudate nucleus became difficult and traces were no longer drawn. The last trace coincided with the border of the anterior and middle third of the thalamus as visualized axially. The medial caudate border was comprised of the cerebrospinal fluid in the lateral ventricle and the lateral border by the frontal white matter anteriorly and the internal capsule more posteriorly. The separation from nucleus accumbens was generally not possible based on visual inspection. The inferior border of the caudate at the level of nucleus accumbens was drawn on the imaginary line connecting the most inferior points of the lateral ventricle and the putamen.
Ventricular Segmentation
The ventricular segmentation approach has been described previously.23 Briefly, a human rater (AEG, intra-rater reliability Cronbach’s Alpha = 0.995) first traced the lateral ventricles of several (2–6, in this case 4) subjects and these traces were then converted into 3D parametric ventricular mesh models, termed atlases. Using fluid registration techniques, each atlas was separately warped to match and, thereby, extract the shape of the lateral ventricle of each new subject’s scan resulting in four lateral ventricle segmentations per subject, which were then averaged to create one final ventricular model that most accurately captures individual anatomy. Averaging four separate segmentations minimizes as much as possible automated labeling errors that occur when only one atlas is used.
Radial Distance Mapping
After modeling the segmented hippocampi, caudate nuclei and lateral ventricles as 3D parametric meshes, we computed the medial core (a medial curve threading down the center of each structure) and the radial distance from the medial core to each surface point for each structure in each subject.24,25 This provides an intuitive measure of the thickness of the structure at each point on the boundary. We then derived 3D group average maps and subjected these to between-group statistical comparisons. The Mini-Mental State Examination (MMSE) and UPDRS Part III:motor subscale scores were entered as covariates in a general linear model predicting the radial distance at each surface point of the mesh models. All significance maps were corrected for multiple comparisons using permutation testing with the predefined threshold of P < 0.01.
Statistical Methods
One-way ANOVA with post-hoc Bonferroni correction for multiple comparisons and chi-squared test were used to test for between-group differences in age, education, age at PD diagnosis, PD duration, Hoehn & Yahr stage,26 UPDRS Part III:motor subscale score, and MMSE score (Table 1). Pearson correlation analyses were used to investigate for possible associations between the volume of our structures of interest, and age, age at disease onset, PD duration and educational level. We used quadratic regression to model potential linear and nonlinear age and education effects on our radial distance maps for each structure of interest and adjusted the radial distance measure, accordingly, if significant effect was present. We used linear regression models to map the association between MMSE and UPDRS Part III:motor subscale and radial distance and in our between-group comparisons. The 3D statistical maps were further subjected to multiple comparisons correction by permutation analyses with the stringent threshold of P < 0.01. This statistical method first defines the area of the map with suprathreshold values (i.e., P < 0.01) and then compares it in 100,000 iterations to the suprathreshold area of 100,000 statistical null distributions, where the pool of predictor variables (e.g. clinical diagnosis, cognitive scores, etc.) is randomly assigned to the study subjects and the significance of the experiment is then defined. The final permutation corrected P-value reflects the likelihood that one’s experimental findings can occur by chance alone. See Thompson et al.27 and Nichols and Holmes28 for more details on the permutation methods.
TABLE 1.
Demographic and volumetric variables
| Variable | NC | PDND | PDMCI | PDD | One-Way ANOVA, P-value |
|---|---|---|---|---|---|
| Age, yr | 73.6 (6.0) | 68.4 (6.8) | 78 (7.8) | 73.4 (7.6) | 0.068 |
| Gender (M:F) | 10:10 | 6:6 | 5:3 | 10:5 | 0.6 |
| Education, yr | 12.1 (4.3) | 11.6 (3.6) | 8.0 (1.5) | 9.8 (4.0) | 0.032 |
| Age at PD presentation, yr | N/A | 55.5 (11.8) | 66.9 (8.6) | 60.1 (12.7) | 0.1 |
| PD duration, yr | N/A | 14.3 (5.1) | 10.5 (4.3) | 13.1 (7.8) | 0.7 |
| Hoehn & Yahr | N/A | 2.3 (0.4) | 2.6 (0.7) | 3.2 (0.7) | 0.007 |
| UPDRS Part III: motor subscale | N/A | 22.7 (5.9) | 34.4 (13.1) | 40.6 (13.2) | 0.006 |
| MMSE | 29.6 (0.7) | 29.4 (0.5) | 26.4 (2.6) | 19.2 (5.1) | <0.001 |
| Left hippocampus, mm3 | 2802 (436) | 2898 (376) | 3127 (442) | 2710 (403) | 0.2 |
| Right hippocampus, mm3 | 2840 (380) | 2855 (653) | 3096 (653) | 2633 (498) | 0.2 |
| Left caudate, mm3 | 2927 (456) | 2987 (523) | 2808 (677) | 2760 (677) | 0.7 |
| Right caudate, mm3 | 2751 (561) | 2812 (374) | 2857 (463) | 2658 (565) | 0.8 |
| Left lateral ventricle, mm3 | 14142 (2329) | 14194 (1939) | 14670 (2728) | 14845 (2341) | 0.8 |
| Right lateral ventricle, mm3 | 15591 (2675) | 15655 (1760) | 16715 (3593) | 17346 (2692) | 0.2 |
RESULTS
The demographics of each group are shown in Table 1. There was a trend-level significant age difference between the PDND and PDMCI groups (P = 0.054). Significant UPDRS Part III:motor subscale (P = 0.006) and Hoehn & Yahr differences (P = 0.007) between the PDND and the PDD groups were also present. We observed the expected differences in MMSE (see Table 1).
Hippocampal Between-group Comparisons
Hippocampal volumetric data (mean, SD) are provided in Table 1. Hippocampal volume correlated with age (left r = −0.34, P = 0.012; right r = −0.37, P = 0.006) but not with age at disease onset, disease duration, or education. The hippocampal quadratic regression models revealed significant age but no education effect. While the uncorrected for age hippocampal between-group 3D comparisons showed significant hippocampal differences between PDND vs. PDMCI in the expected direction, this effect disappeared after controlling for age.
Caudate Between-group Comparisons
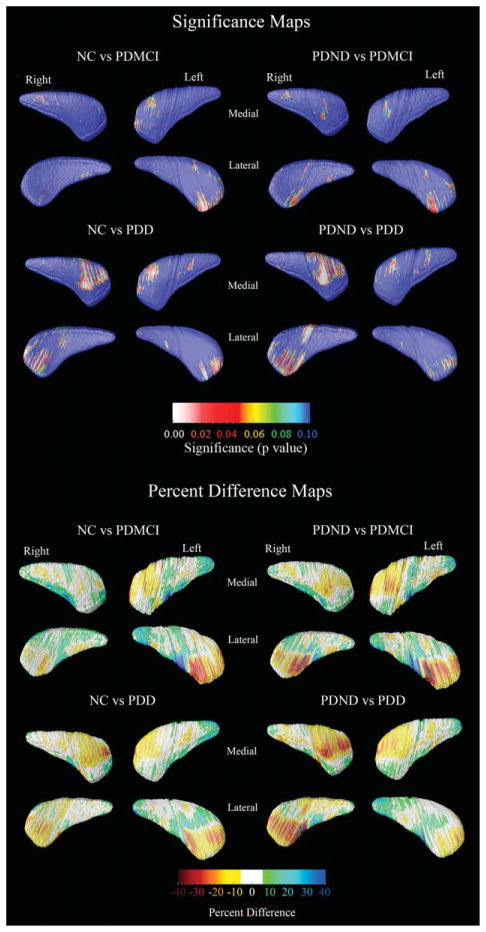
Caudate volumetric data (mean, SD) are provided in Table 1. There were no correlations between caudate volumes and age, age of disease onset, disease duration, or educational level. Even so, the 3D quadratic regression models revealed focal areas of significant age effects. Education did not show an effect on the 3D caudate models. The 3D age-adjusted between-group caudate statistical maps are presented in Figure 1. After multiple comparison correction with permutation testing, significant differences were detected in the left lateral (Pcorrected = 0.034) and medial (Pcorrected = 0.034) and right medial (Pcorrected = 0.023) caudate between NC and PDD and right medial caudate between PDND and PDD subjects (pcorrected = 0.034). Trend-level significant differences were seen in the left lateral caudate in the NC vs. PDMCI comparison (Pcorrected = 0.07) and the right lateral caudate in the NC vs. PDND comparison (Pcorrected = 0.09). The quantitative maps shown in the bottom portion of Figure 1 demonstrate that those areas where significant between-group differences were seen show 10–40% smaller caudate radial distance, on average, in the PDD group versus the PDND and the NC groups, respectively. A few caudate areas showed between-group differences in a direction opposite to our predefined hypotheses (green and blue regions in the percent difference caudate maps in the bottom portion of Fig. 1). However, these effects were not significant after stringent multiple comparison correction with permutation testing.
FIG. 1.
3D statistical and quantitative between-group caudate maps. The statistical maps (top) show regions where statistically significant between-group differences are seen. The red and white colors denote areas where the P-value is equal to or less than 0.05 and the dark blue colors denote P-values of 0.1 or higher. The quantitative maps (bottom) show the magnitude of between-group differences (in %).
Ventricular Between-group Comparisons
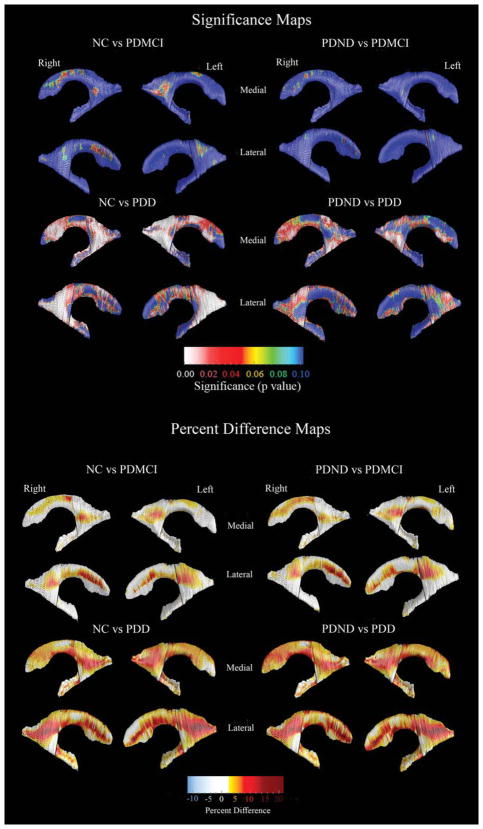
Ventricular volumetric data (mean, SD) are provided in Table 1. There were no correlations between ventricular volumes and age, age of disease onset, disease duration, or education. Even so, the 3D quadratic regression models revealed focal areas of significant age effects. Education did not show an effect on the 3D ventricular models. The 3D age-adjusted between-group ventricular statistical maps are presented in Figure 2. Following correction for multiple comparisons via permutation testing the PDD group showed significantly larger left (Pcorrected = 0.011) and right (Pcorrected = 0.004) ventricles, compared to the NC group. The group differences had greatest effect sizes in the posterior parts of the lateral ventricles (body/ occipital horn left Pcorrected = 0.00016, right Pcorrected = 0.00053; superior horn left Pcorrected = 0.023, right Pcorrected = 0.012; inferior horn left Pcorrected = 0.012, right Pcorrected = 0.0024). When compared to the PDND group, the PDD group showed significant right and trend-level left ventricular enlargement (left Pcorrected = 0.076, right Pcorrected = 0.05), where the results were driven by differences in the posterior portions of the lateral ventricles (body/occipital horn left Pcorrected = 0.031, right Pcorrected = 0.014). The PDD group showed significant differences in the right and trend-level significant changes in the left posterior ventricular portions (body/occipital horn left Pcorrected = 0.0061, right Pcorrected = 0.045), compared to PDMCI. There were no significant differences in the PDMCI vs. NC and PDMCI vs. PDND comparisons. The quantitative maps shown in the bottom portion of Figure 2 demonstrate that the areas with significant between-group differences show 7–25% greater ventricular enlargement in the PDD group versus the PDND and the NC control groups, respectively. There were no areas with effects in a direction opposite to our hypothesis.
FIG. 2.
3D statistical and quantitative between-group ventricular maps. The statistical maps (top) show regions where statistically significant between-group differences are seen. The red and white colors denote areas where the P-value is equal to or less than 0.05 and the dark blue colors denote P-values of 0.1 or higher. The quantitative maps (bottom) show the magnitude of between-group differences (in %).
Cognitive and Motor Correlations
Using linear regression, we investigated the strength of the association between two disease-associated measures—the MMSE (measuring global cognitive decline) and the UPDRS Part III: motor subscale (measuring motor symptom severity)—and caudate and hippocampal atrophy and ventricular enlargement. MMSE and UPDRS Part III: motor subscale scores did not show significant correlations with hippocampal atrophy. The caudate and ventricular clinical correlation maps are shown in Figures 3 and 4, respectively.
FIG. 3.
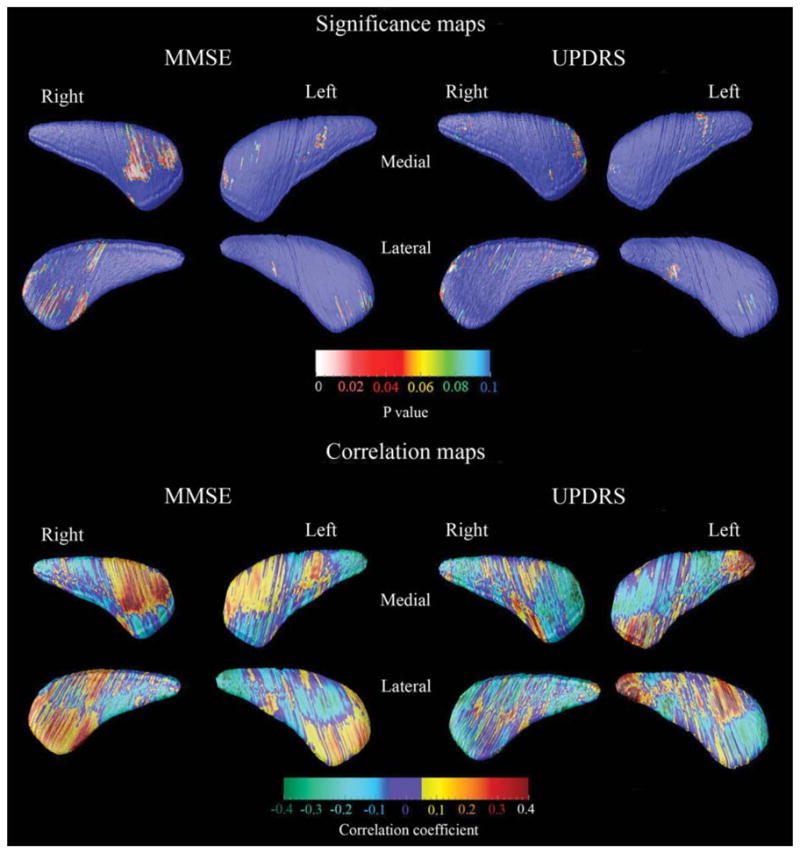
3D caudate clinical covariate maps. For the significance maps (top two rows) the red and white colors denote areas where the P-value is equal to or less than 0.05 and the dark blue colors denote P-values of 0.1 or higher. The correlation maps (bottom two rows) show the strength of the correlations between radial distance (or caudate thickness) and MMSE or UPDRS Part III:motor subscale at each surface point of the caudate model.
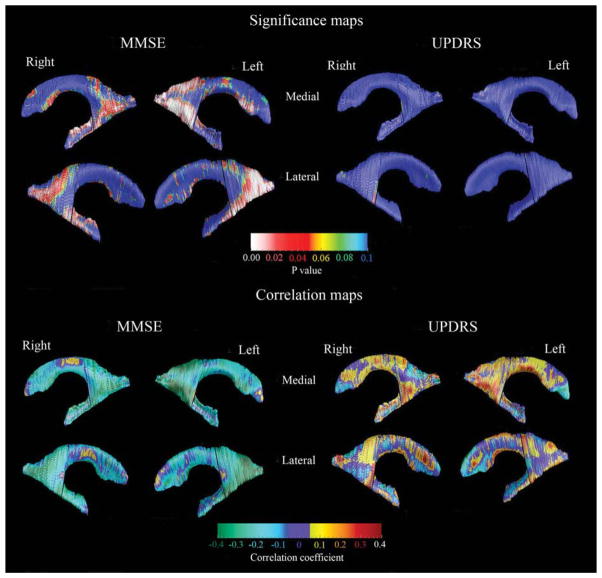
FIG. 4.
3D ventricular clinical covariate maps. For the significance maps (top two rows), the red and white colors denote areas where the P-value is equal to or less than 0.05 and the dark blue colors denote P-values of 0.1 or higher. The correlation maps (bottom two rows) show the strength of the correlations between radial distance (or ventricular expansion) and MMSE or UPDRS Part III:motor subscale scores at each surface point of the caudate model.
We found regionally pronounced positive correlations between MMSE and the head of the caudate nucleus, however these differences showed only trend-level significance (P < 0.098) after multiple comparison correction with permutation testing at P < 0.01 threshold (Fig. 3, left top and bottom panels). UPDRS Part III:motor subscale scores did not show significant associations with caudate radial distance (Fig. 3, right top and bottom panel).
We found significant/trend-level significant positive associations between MMSE and enlargement of the inferior and posterior lateral ventricle horns (body/occipital horn on the left Pcorrected = 0.0027, right Pcorrected = 0.053; inferior horn, left Pcorrected = 0.062, right Pcorrected = 0.018; Fig. 4, left top and bottom panels). Although local correlations between UPDRS Part III:-motor subscale and ventricular radial distance also were observed, these did not remain significant after correction for multiple comparisons (Fig. 4, right top and bottom panels).
DISCUSSION
This is the first 3D mapping study to examine the structural alterations of the caudate nuclei, hippocampi, and lateral ventricles in PD subjects with and without dementia. PD subjects with cognitive decline show preferential atrophy of the head of the caudate nuclei (Fig. 1) and enlargement in the posterior regions of the lateral ventricles (Fig. 2). The head of the caudate nucleus is part of the dorsolateral prefrontal circuit (DLPFC).29 Disruption of the DLPFC could lead to prefrontal cognitive deficits and impaired memory. Lesions of the head of the caudate nucleus can cause a dysexecutive syndrome, disturbance of attention, and impaired recent and remote memory.30,31 One group reported an association between caudate atrophy and executive dysfunction in PD.32 Figure 3 suggests that cognitive decline correlates with atrophy of the caudate head, although these effects showed only trend-level significance following the predefined stringent correction for multiple comparisons and should only be interpreted with caution.
Ventricular expansion has received considerable attention in the AD literature.33,34 Ventricular volume33 and its rate of change34 have been associated with future cognitive decline to AD-type MCI and dementia in NC. Our 3D analyses suggest that PDD is likewise associated with lateral ventricle enlargement, and that these changes are most prominent posteriorly (Fig. 2). The expansion of the posterior portions of the lateral ventricles is likely due to structural changes in the parietal and occipital lobes and/or their connections with other brain regions. In contrast the expansion of the frontal horns likely reflects atrophy of the basal ganglia including the caudate nuclei, as well as gray and white matter changes of the frontal lobe. Further evaluation for potential gray and white matter atrophy of the frontal, occipital and parietal lobes, as well as the globus pallidus and putamen will be necessary to fully evaluate these findings. The observed strong association of MMSE and the posterior portions of the lateral ventricles (Fig. 4) further supports this conclusion.
We hypothesized that we would find hippocampal atrophy but did not detect it. There may be subtle disease-associated hippocampal differences that require a larger sample to identify. In addition, only a global measure of cognition was used. A more detailed assessment of memory and other cognitive domains using more sensitive neuropsychological tools may increase our power to detect disease-related associations between cognition and hippocampal atrophy.
Several strengths and limitations of our study should be recognized. Major strengths of the study include the well-characterized PD patient cohort. The state-of-the-art imaging technique is another strength, as it can identify focal regionally specific disease-associated changes that are more difficult to identify with more conventional analytic techniques. In addition, surface mapping techniques have proven more sensitive to subtle disease-associated effects relative to ROI-based volumetric analyses.35–38 One limitation of our study is its small sample size, which restricted our power to achieve statistical significance in several of the analyses. However, larger studies, presently underway, will provide the necessary statistical power to detect or refute potential between group differences. Subjects did not receive an identical neuropsychological battery which limited our ability to assess more specific associations between structural alterations and cognitive decline. The age and education mismatch observed between the groups is another relative limitation that we addressed by scrutinizing the age and education effects on each structure of interest and including the variable showing statistically significant associations (i.e., age) as a confounder in all statistical comparisons. We did not observe statistically significant between-group sex differences in our study (P = 0.6), but we acknowledge that if sufficiently large samples were available, all imaging studies would ideally model gender effects. In view of our small group sample sizes, and to avoid unnecessarily losing a degree of freedom of the statistical models, we chose not to include adjustment for sex. Future studies will be needed to investigate possible associations between visuospatial performance and ventricular enlargement, memory and hippocampal atrophy, or executive dysfunction and anterior caudate atrophy.
Acknowledgments
This study was generously supported by NIA K23 AG026803 and the Turken Foundation (to LGA); NIA AG16570 (to JLC, LGA and PMT); NIBIB EB01651, NLM LM05639, NCRR RR019771 (to PMT); and NIMH R01 MH071940, NCRR P41RR013642 and NIH U54 RR021813 and the Western Norway Regional Health Authority. The authors have no other disclosures/competing interests.
Footnotes
Potential conflict of interest: None reported
Author Roles: Liana Apostolova was involved in conception, organization, and execution of the study; design, execution, and review of the statistical analyses; and writing and editing of the manuscript. Mona Beyer was involved in conception and organization of the study; data collection; design and review of the statistical analyses; and writing and editing of the manuscript. Amity Green was involved in execution of the study and statistical analyses, writing and editing of the manuscript. Kristy Hwang was involved in execution of the study and statistical analyses, writing and editing of the manuscript. Jonathan Morra was involved in execution of the study and editing of the manuscript. Yi-Yu Chou was involved in execution of the study and editing of the manuscript. Christina Avedissian was involved in execution of the study and editing of the manuscript. Dag Aarsland was involved in conception and organization of the study; data collection; review of the statistical analyses; editing, review, and critique of the manuscript. Carmen Janvin was involved in data collection; review of the statistical analyses; and editing, review, and critique of the manuscript. Jan Larsen was involved in data collection; review of the statistical analyses; and editing, review, and critique of the manuscript. Jeffrey Cummings was involved in conception of the study; review of the statistical analyses; and editing, review, and critique of the manuscript. Paul Thompson was involved in organization and execution of the study; review of the statistical analyses; and writing, editing, review, and critique of the manuscript.
References
- 1.Jellinger KA. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci. 2009;24:114–125. doi: 10.1159/000197890. [DOI] [PubMed] [Google Scholar]
- 2.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer MK, Larsen JP, Aarsland D. Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology. 2007;69:747–754. doi: 10.1212/01.wnl.0000269666.62598.1c. [DOI] [PubMed] [Google Scholar]
- 4.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 5.Junque C, Ramirez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord. 2005;20:540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 6.Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64:861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- 7.Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75:1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camicioli RM, Korzan JR, Foster SL, et al. Posterior cingulate metabolic changes occur in Parkinson’s disease patients without dementia. Neurosci Lett. 2004;354:177–180. doi: 10.1016/j.neulet.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 9.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 10.Kassubek J, Juengling FD, Hellwig B, Spreer J, Lucking CH. Thalamic gray matter changes in unilateral Parkinsonian resting tremor: a voxel-based morphometric analysis of 3-dimensional magnetic resonance imaging. Neurosci Lett. 2002;323:29–32. doi: 10.1016/s0304-3940(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 11.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 12.Alegret M, Junque C, Pueyo R, et al. MRI atrophy parameters related to cognitive and motor impairment in Parkinson’s disease. Neurologia. 2001;16:63–69. [PubMed] [Google Scholar]
- 13.Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006;58:256–262. doi: 10.1227/01.NEU.0000194845.19462.7B. discussion. [DOI] [PubMed] [Google Scholar]
- 14.Huber SJ, Shuttleworth EC, Christy JA, Chakeres DW, Curtin A, Paulson GW. Magnetic resonance imaging in dementia of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1989;52:1221–1227. doi: 10.1136/jnnp.52.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen JP, Dupont E, Tandberg E. Clinical diagnosis of Parkinson’s disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol Scand. 1994;89:242–251. doi: 10.1111/j.1600-0404.1994.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 16.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 17.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The cooperative multicentric group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 19.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 20.Apostolova LG, Steiner CA, Akopyan GG, Toga AW, Cummings JL, Thompson PM. 3D gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer’s disease. Arch Neurol. 2007;64:1489–1495. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narr KL, Van Erp TG, Cannon TD, et al. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- 22.Morra JH, Tu Z, Apostolova LG, et al. Validation of a fully automated 3D hippocampal segmentation method using subjects with Alzheimer’s disease, mild cognitive impairment and elderly controls. Neuroimage. 2008;43:59–68. doi: 10.1016/j.neuroimage.2008.07.003. Erratum in: Neuroimage 2009 Feb 15;44(4):1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou YY, Lepore N, De Zubicaray GI, et al. Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer’s disease. Neuroimage. 2008;40:615–630. doi: 10.1016/j.neuroimage.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apostolova LG, Dinov ID, Dutton RA, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129:2867–2873. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- 25.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 26.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PM, Hayashi KM, De Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–354. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- 31.Richfield EK, Twyman R, Berent S. Neurological syndrome following bilateral damage to the head of the caudate nuclei. Ann Neurol. 1987;22:768–771. doi: 10.1002/ana.410220615. [DOI] [PubMed] [Google Scholar]
- 32.Camicioli R, Gee M, Bouchard TP, et al. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine-refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Carmichael OT, Kuller LH, Lopez OL, et al. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:389–397. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts future decline to Alzheimer’s dementia in cognitively normal subjects. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.08.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bearden CE, Thompson PM, Dutton RA, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepore N, Shi Y, Lepore F, et al. Pattern of hippocampal shape and volume differences in blind subjects. Neuroimage. 2009;46:949–957. doi: 10.1016/j.neuroimage.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolson R, Devito TJ, Vidal CN, et al. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Res. 2006;148:11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]