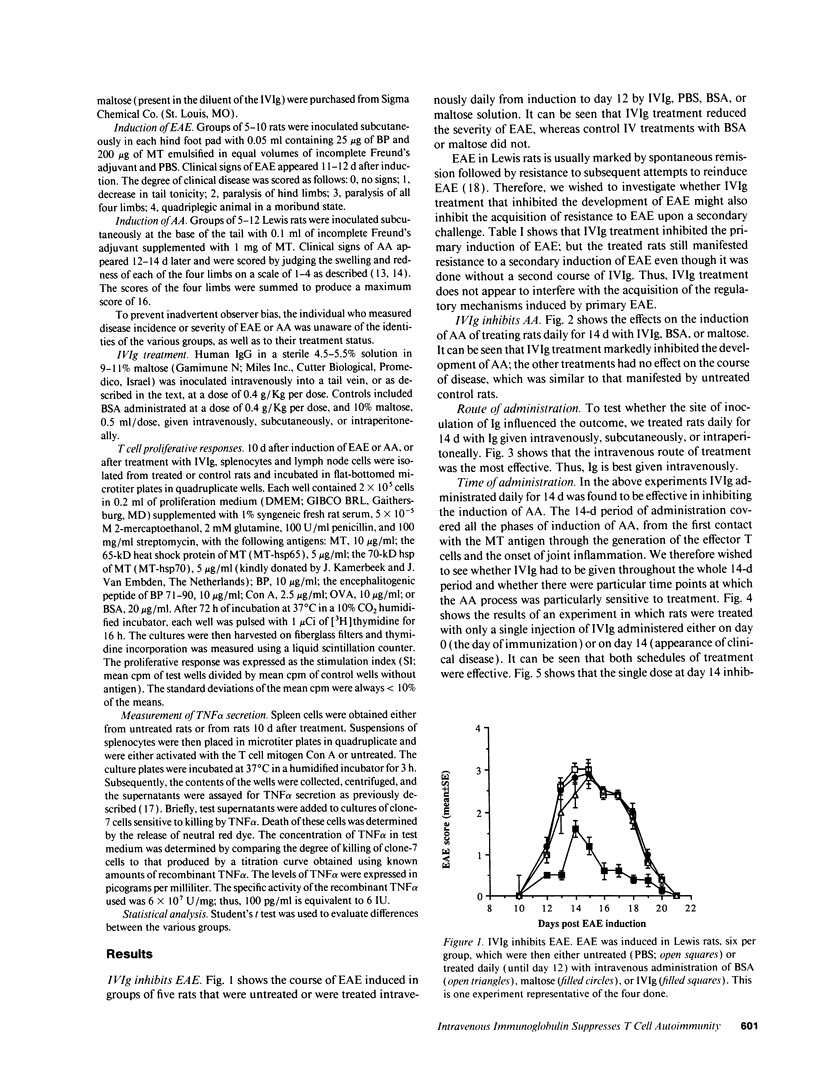
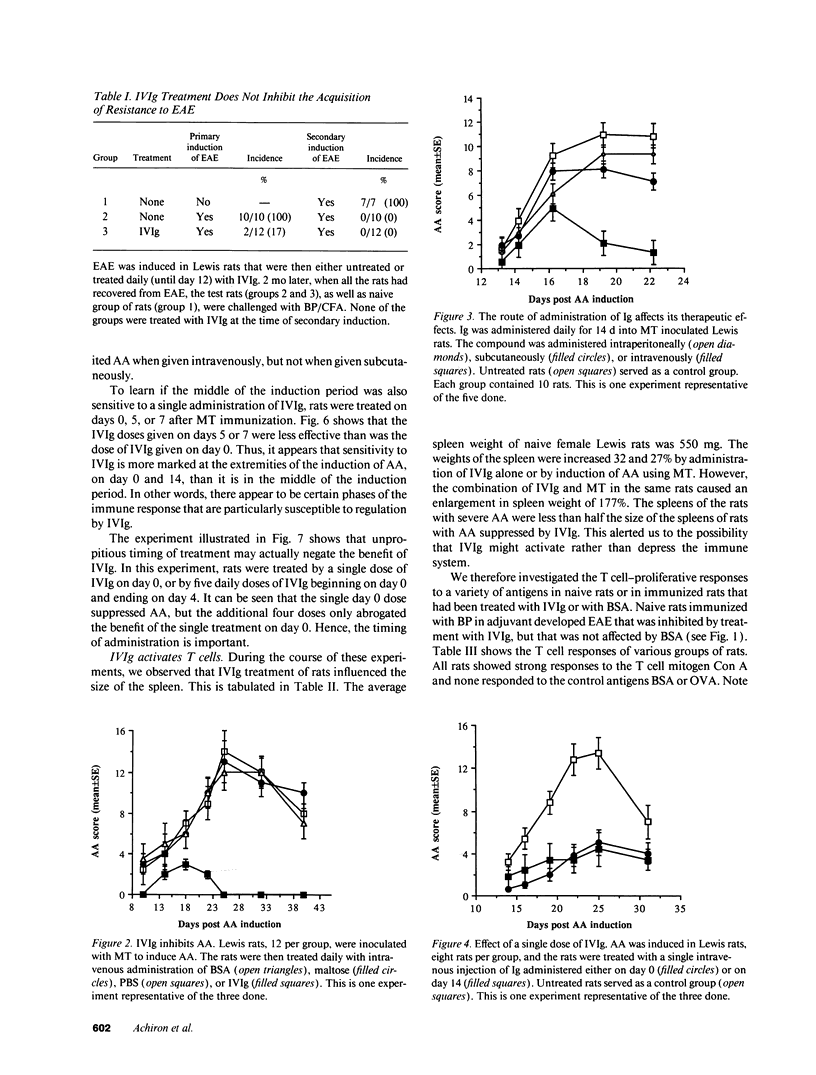
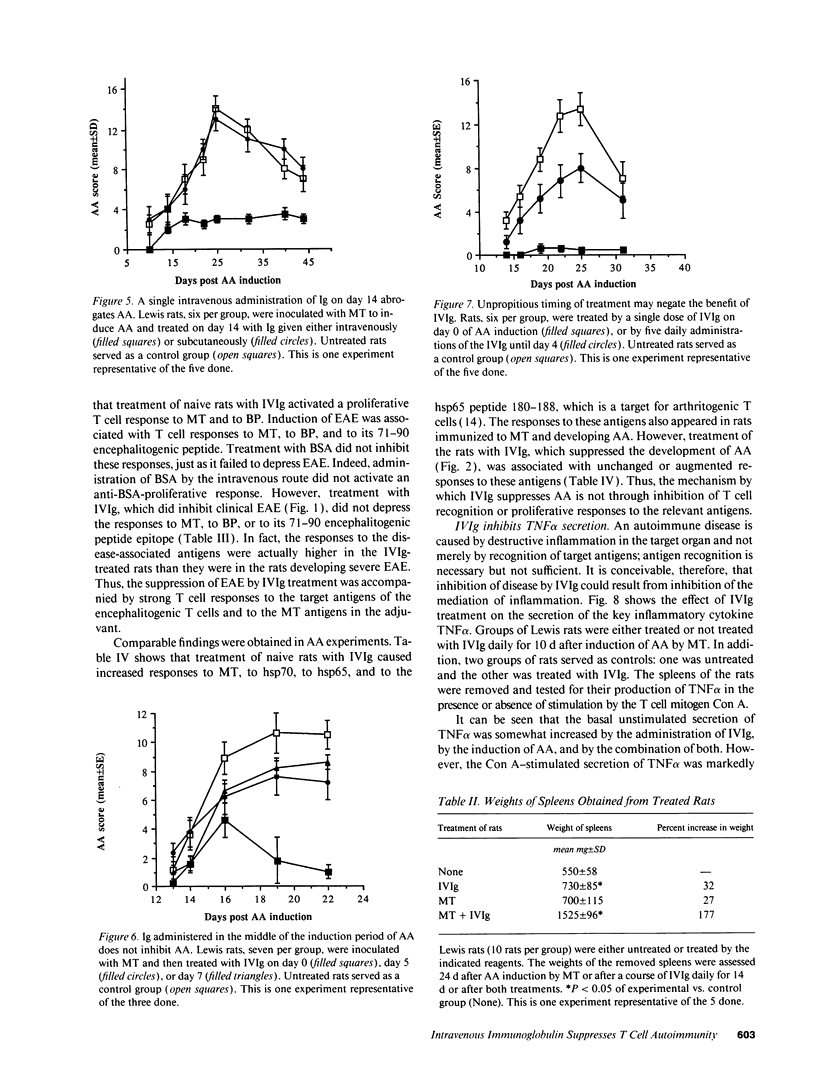
Abstract
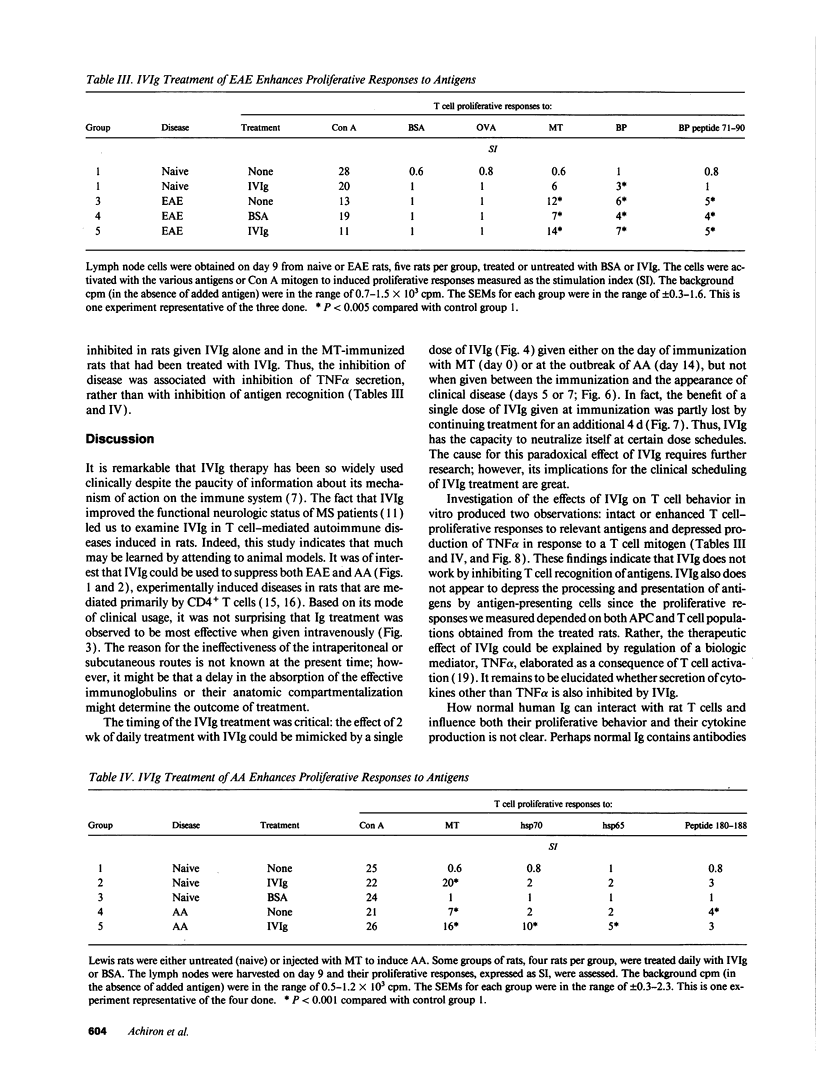
It has been reported previously that intravenous administration of normal human immunoglobulins (IVIg) to human patients can suppress the clinical signs of certain autoimmune diseases. However, the mechanism(s) by which normal Ig interferes with the various disorders and the scheduling of treatment have been poorly delineated. To study these questions, we examined IVIg treatment of two experimentally induced T cell autoimmune diseases in rats: experimental autoimmune encephalomyelitis (EAE) and adjuvant arthritis (AA). We now report that IVIg treatment (0.4 g/kg) inhibited the active induction of both EAE and AA, and that this treatment did not affect the acquisition of resistance to reinduction of EAE. The importance of the site of administration and schedule of treatment were studied in the AA model. Ig was effective when given intravenously, but not when administrated subcutaneously or intraperitoneally. IVIg treatment was effective when given daily from immunization to outbreak of disease; but it was also effective when given once at the time of immunization or once 2 wk after induction of AA, just at the clinical outbreak of disease. Administration of IVIg between immunization and outbreak of AA was less effective. Prevention of disease by IVIg occurred despite the presence of T cell reactivity to the specific antigens in the disease. In fact, IVIg administrated to naive rats activated T cell reactivity to some self-antigens. Nevertheless, IVIg treatment led to decreased production of the inflammatory cytokine TNF alpha. Thus, IVIg treatment may exert its therapeutic power not by inhibiting T cell recognition of self-antigens, but by inhibiting the biological consequences of T cell recognition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Achiron A., Pras E., Gilad R., Ziv I., Mandel M., Gordon C. R., Noy S., Sarova-Pinhas I., Melamed E. Open controlled therapeutic trial of intravenous immune globulin in relapsing-remitting multiple sclerosis. Arch Neurol. 1992 Dec;49(12):1233–1236. doi: 10.1001/archneur.1992.00530360031013. [DOI] [PubMed] [Google Scholar]
- Arsura E. L., Bick A., Brunner N. G., Grob D. Effects of repeated doses of intravenous immunoglobulin in myasthenia gravis. Am J Med Sci. 1988 May;295(5):438–443. doi: 10.1097/00000441-198805000-00005. [DOI] [PubMed] [Google Scholar]
- Basta M., Kirshbom P., Frank M. M., Fries L. F. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989 Dec;84(6):1974–1981. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Spontaneous remission and acquired resistance to autoimmune encephalomyelitis (EAE) are associated with suppression of T cell reactivity: suppressed EAE effector T cells recovered as T cell lines. J Immunol. 1982 Mar;128(3):1450–1457. [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Berkman S. A., Lee M. L., Gale R. P. Clinical uses of intravenous immunoglobulins. Semin Hematol. 1988 Apr;25(2):140–158. [PubMed] [Google Scholar]
- Cohen I. R. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992 Dec;13(12):490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. The cognitive principle challenges clonal selection. Immunol Today. 1992 Nov;13(11):441–444. doi: 10.1016/0167-5699(92)90071-E. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M. Manipulating the immune system with immune globulin. N Engl J Med. 1992 Jan 9;326(2):107–116. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Kleyweg R. P., van der Meché F. G., Meulstee J. Treatment of Guillain-Barré syndrome with high-dose gammaglobulin. Neurology. 1988 Oct;38(10):1639–1641. doi: 10.1212/wnl.38.10.1639. [DOI] [PubMed] [Google Scholar]
- Lider O., Reshef T., Beraud E., Ben-Nun A., Cohen I. R. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 1988 Jan 8;239(4836):181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- Rossi F., Dietrich G., Kazatchkine M. D. Anti-idiotypes against autoantibodies in normal immunoglobulins: evidence for network regulation of human autoimmune responses. Immunol Rev. 1989 Aug;110:135–149. doi: 10.1111/j.1600-065x.1989.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Wallach D. Preparations of lymphotoxin induce resistance to their own cytotoxic effect. J Immunol. 1984 May;132(5):2464–2469. [PubMed] [Google Scholar]
- van Doorn P. A., Brand A., Strengers P. F., Meulstee J., Vermeulen M. High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study. Neurology. 1990 Feb;40(2):209–212. doi: 10.1212/wnl.40.2.209. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van der Meché F. G., Schmitz P. I. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. 1992 Apr 23;326(17):1123–1129. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]