Abstract
OBJECTIVE
Several co-infections have been shown to impact the progression of HIV-1 infection. We sought to determine if treatment of helminth co-infection in HIV-1 infected adults impacted markers of HIV-1 disease progression.
DESIGN
To date there have been no randomized trials to examine the effects of soil-transmitted helminth eradication on markers of HIV-1 progression.
METHODS
A randomized, double-blind, placebo-controlled trial of albendazole (400mg daily for three days) in antiretroviral-naïve HIV-1 infected adults (CD4 >200 cells/mm3) with soil-transmitted helminth infection was conducted at ten sites in Kenya (Clinical Trials.gov NCT00130910). CD4 and plasma HIV-1 RNA levels at 12 weeks following randomization were compared in the trial arms using linear regression, adjusting for baseline values.
RESULTS
Of 1,551 HIV-1 infected individuals screened for helminth-infection, 299 were helminth-infected. 234 adults were enrolled and underwent randomization and 208 individuals were included in intent-to-treat analyses. Mean CD4 count was 557 cells/mm3 and mean plasma viral load was 4.75 log10 copies/mL at enrolment. Albendazole therapy resulted in significantly higher CD4 counts among individuals with Ascaris lumbricoides infection after 12 weeks of follow up (+109 cells/mm3; 95% CI +38.9 to +179.0, p=0.003) and a trend for 0.54 log10 lower HIV-1 RNA levels (p=0.09). These effects were not seen with treatment of other species of soil-transmitted helminths.
CONCLUSIONS
Treatment of A. lumbricoides with albendazole in HIV-1 co-infected adults resulted in significantly increased CD4 counts during 3-month follow-up. Given the high prevalence of A. lumbricoides infection worldwide, deworming may be an important potential strategy to delay HIV-1 progression.
Keywords: HIV-1 progression, helminth, co-infection
The majority of HIV-1 infected individuals in settings with high HIV-1 seroprevalence do not yet meet criteria for initiation of antiretroviral therapy. Interventions to delay HIV-1 progression are needed to maximize the health of these individuals and delay the time until initiation of antiretroviral treatment. There is evidence from observational studies that treatment of some co-infections may delay HIV-1 disease progression.
Soil-transmitted helminths are among the most prevalent infections of humans worldwide. More than 2 billion people are estimated to be infected with soil-transmitted helminths and the geographical distribution of these infections overlaps considerably with regions of high HIV-1 seroprevalence[1, 2]. Helminth co-infection has been hypothesized to be one factor driving the HIV-1 epidemic in Africa[3, 4]. A randomized clinical trial of schistosomiasis treatment in 130 HIV-1 infected individuals found that treatment was associated with significantly lower rise in plasma HIV-1 RNA[5]. While suggestive of potential associations between helminth infection and HIV-1 progression, the pathogenesis of schistosomiasis, and consequently its interactions with HIV-1, may differ from other helminths. Several observational studies have yielded conflicting data regarding the association between soil-transmitted helminth treatment and HIV-1 progression[6]. To date, no randomized clinical trials have been conducted to determine the effect of treatment of soil-transmitted helminths on HIV-1 progression.
If helminth eradication can delay HIV-1 progression, it is a simple intervention that could be widely and rapidly implemented, with significant public health impact. We conducted a randomized, double-blind, placebo-controlled trial to determine whether albendazole treatment in HIV-1 and helminth co-infected individuals impacts markers of HIV-1 disease progression, specifically CD4 count and plasma HIV-1 RNA.
METHODS
Study Design
Antiretroviral-naïve HIV-1 seropositive adults with evidence of co-infection with albendazole-treatable soil-transmitted helminths were eligible for enrollment. Eligible individuals were randomized to receive either 400 mg albendazole or placebo once daily for three consecutive days and were followed up after 12 weeks. The study was approved by the Kenya Medical Research Institute Ethical Review Board and the University of Washington Institutional Review Board. All participants provided written informed consent. The study was registered under Clinical Trials Registration identifier: NCT00130910.
Participants
Study participants were recruited from existing HIV-1 Care and Treatment programs at 10 sites geographically dispersed throughout Kenya using a mobile study team. Study sites were the Kibera AMREF/CDC Clinic, Homa Bay District Hospital, Kerugoya District Hospital, Coptic Hope Clinic (Nairobi), Mbagathi District Hospital, Thika District Hospital, Kisumu District Hospital, Kisii District Hospital, Machakos District Hospital and the Kilifi District Hospital. Subjects were eligible for screening if they were HIV-1 seropositive, at least 18 years of age, not pregnant and were not eligible for initiation of ART based on WHO guidelines (CD4 <200 cells/mm3, any stage 4 and some stage 3 disease)[7]. Exclusion criteria also included having ever used antiretroviral drugs, having taken medicine for helminth infection in the preceding 6 months, evidence of active tuberculosis or TB treatment in the past 3 months, and clinical signs of severe anemia.
Trial Procedures
Potentially eligible study participants were identified at each site by clinic staff and referred for helminth screening. At screening visits, each participant was informed of the aims and procedures of the study and assessed for eligibility. Female participants were asked to provide a fresh urine sample for β-HCG testing. Men and β-HCG negative women were asked to provide stool for analysis. Baseline demographic and medical history was obtained from participants at the time of screening. Participants were provided with stool collection containers and instructions on specimen collection, and requested to collect stool within 6 hours of the screening visit. Individuals with evidence of albendazole-treatable pathogenic soil-transmitted helminths on microscopy were invited to be enrolled in the trial. Individuals with schistosomiasis or infection with Taenia species were treated with open label praziquantel (and albendazole if co-infected with other helminths) and were not enrolled. Individuals with no evidence of helminth infection were counseled on basic hygiene and avoidance of helminth exposure and were not enrolled in the randomized trial.
Individuals with documented soil-transmitted helminth infections treatable with albendazole (Ascaris lumbricoides, hookworm species, or Trichuris trichiura) were invited to attend an additional informed consent session at which time the aims and the procedures of the trial were reviewed. Individuals were enrolled in the trial after written informed consent. A medical or clinical officer performed baseline clinical examinations and collected additional clinical and demographic information on all participants at enrollment. Blood was collected for repeat HIV-1 serologic testing, measurement of CD4 lymphocytes, and plasma HIV-1 RNA levels. Participants were randomly assigned to two groups using a 1:1 allocation scheme with block randomization of 30 patients and following a random-allocation list generated independently. Pre-labeled, sequentially numbered treatment packs were used. Both the active drug (albendazole) and an identical appearing placebo were provided by the drug manufacturer. Investigators, clinic staff and patients were blinded to study-group assignment. The first dose of study medication was taken at enrollment and was directly observed. The remaining doses were dispensed to the patient. Enrolled participants were scheduled to return for follow up at a single visit 12 weeks after randomization. Participants were given 2 stool collection vials (plain and preservative) and asked to return for the follow up visit with a stool sample collected into both vials within 6 hours of the appointment.
At the 12 week follow up visit all female participants were asked to provide an additional fresh urine sample for β-HCG testing and blood was collected for CD4 lymphocytes and plasma HIV-1 RNA levels. Participants with evidence of helminth infection at the 12 week visit were treated with open label albendazole and/or praziquantel as indicated regardless of randomization arm. Participants who did not provide stool for analysis at the 12 week visit were presumptively treated with albendazole 400mg a day for three days. Women who were pregnant at the follow up visit and who were either positive for helminths by stool analysis or who did not provide stool for analysis were referred to the antenatal care clinic at the site where they were enrolled for management.
Laboratory Analysis
All randomized participants underwent repeat HIV-1 serological testing for HIV-1 using Determine™ rapid test qualitative immunoassay (Abbott, Japan). The CD4 lymphocyte count was determined using Multiset™ software on a FACSCalibur machine (Becton Dickinson, USA).
Plasma HIV-1 RNA was quantified using the Gen-Probe HIV-1 viral load assay, which has been shown to quantify the subtypes of HIV-1 prevalent in Kenya[8].
Each participant provided stool samples in a plain collection vial (AlphaTec, USA) and a vial containing preservative (Protofix™CLR, AlphaTec, USA). Each stool sample was processed and evaluated using wet-preparation, Kato-Katz and formol-ether concentration techniques by an experienced laboratory technician. The presence of protozoa or helminth eggs was recorded and the burden of infection based on number of eggs per gram of stool was calculated according to WHO criteria[9].
Study Outcomes
The primary study outcomes were CD4 counts and log10 plasma HIV-1 RNA levels in the two study arms. Secondary outcomes included CD4 counts and log10 plasma HIV-1 RNA levels in the two arms stratified by helminth species.
Statistical analysis
Based on an estimated difference in CD4 count of 40 cells/mm3 between the groups and using data from a previously accrued observational HIV-1 cohort in Kenya we determined that 200 individuals were required to detect a difference of 40 cells/mm3 between the two study arms using a two sided test, with an estimated standard deviation of 100 cells/mm3, a power of 80% and a 5% type I error, allowing for 15% loss to follow-up.[10]. This sample size also gave 90% power to detect a difference of 0.5 log10 copies/mL of plasma HIV-1 RNA between the groups using a two sided test, assuming a standard deviation of 1.1 log10 copies/mL, and a 5% type I error.
Analyses were conducted with SPSS version 15 (Chicago, USA). Following a data analysis plan developed prior to unblinding, a modified intent-to-treat analysis was conducted. Individuals found to be HIV-1 seronegative on repeat testing or individuals who had initial CD4 counts of less than 200 cells/mm3 but whose CD4 count results were unavailable at randomization were excluded from analysis. Viral loads were log10 transformed before analysis. Baseline characteristics of the participants were compared between the two arms using Chi-square tests for categorical variables and Student’s t-tests for normally distributed continuous variables.
Primary analyses were conducted to evaluate the effect of treatment on the follow up CD4 count and log10 plasma HIV-1 RNA levels. Linear regression (ANCOVA) was performed adjusting for initial CD4 count for the CD4 count outcome, and log10 plasma HIV-1 RNA for the plasma HIV-1 RNA outcome[11]. Intent-to-treat analyses were repeated after stratifying by infecting helminth species. All individuals with a given helminth species (whether single or multiple species) were included in each stratified analysis and a separate analysis was conducted on those individuals with multiple species of helminth co-infection. Analysis of variance was conducted to determine the interaction between each species of helminth on CD4 count outcomes.
Results
Study Population
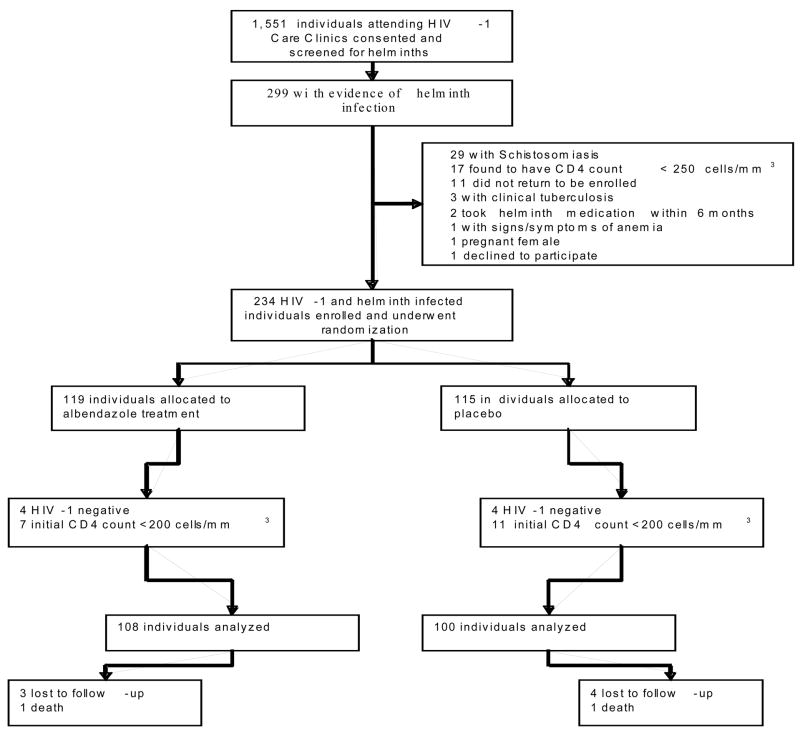
Between March 2006 and June 2007, we screened 1,551 individuals for helminth co-infection. Of these individuals, a total of 299 (19.3%) were infected with at least one species of helminth. A total of 29 individuals (1.9%) had documented schistosomiasis infection, of which 8 (27.6%) were also infected with other soil-transmitted helminths. These individuals were not eligible for inclusion in the study, were provided open-label treatment, and not enrolled. Two hundred and thirty-five individuals were eligible to participate in the study and two hundred and eight individuals were included in the final intent-to treat analysis; 108 randomized to treatment and 100 to placebo (Figure 1). The mean age of the participants was 34 years (range 18–65) and 83.1% were female. There were no significant differences at enrollment between the randomization arms with regard to demographic or social characteristics (Table 1). At enrollment, the mean CD4 count was 557 cells/mm3 (SD 273) and the mean plasma HIV-1 RNA level was 4.75 log10 copies/mL (SD 1.0). There were no significant differences between baseline CD4 counts or plasma HIV-1 RNA levels between the study arms in the intent to treat population or when stratified by species of infecting helminth (Table 2).
Figure 1.
Flow chart of study enrollment
Table 1.
Characteristics of the study participants at baseline
| Albendazole (mean (SD) or %) n=108 |
Placebo (mean (SD) or %) n=100 |
||
|---|---|---|---|
| Age, yearsa | 35 (9.6) | 34 (8.8) | |
| Female | 86.0 | 80.0 | |
| Clinic Location | Nairobi (urban) | 31.5 | 32.0 |
| Greater-Nairobi (semiurban) | 22.2 | 24.0 | |
| Western Kenya (rural) | 24.1 | 20.0 | |
| Coastal (rural) | 22.2 | 24.0 | |
| Educationb | None | 20.2 | 12.4 |
| Primary | 51.9 | 62.9 | |
| Secondary | 22.1 | 19.6 | |
| Post-Secondary | 5.8 | 3.1 | |
| No. Rooms in homec | 1.9 (1.0) | 2.1 (1.1) | |
| Water Sourcec | Piped water in home | 8.6 | 8.2 |
| Communal water source | 53.4 | 50.0 | |
| Lake, river or pool | 21.9 | 24.5 | |
| Other | 16.1 | 17.3 | |
| Sanitationc | Flush toilet in home | 5.7 | 4.1 |
| Shared flush toilet | 6.7 | 8.2 | |
| Pit Latrine for single home | 35.2 | 41.8 | |
| Shared pit latrine | 37.1 | 31.6 | |
| No toilet or latrine | 14.3 | 14.3 | |
| Employed outside homec | 57.7 | 48.0 | |
| No. Children in homec | 1.8 (1.5) | 1.9 (1.5) | |
| Baseline helminth infection status | Ascaris lumbricoides | 24.1 | 28.0 |
| Hookworm species | 70.4 | 72.0 | |
| Trichuris trichiura | 11.1 | 12.0 | |
| Mixed infections | 6.5 | 12.0 | |
(n=105, 100)
(n=104, 97)
(n=105, 98)
Table 2.
Baseline laboratory values by arm.
| Baseline Laboratory measures, mean (SD) | Albendazole | Placebo | |
|---|---|---|---|
| ITT Group | n=108 | n=100 | |
| CD4 count (cells/mm3) | 535.9 (244.8) | 580.5 (299.1) | |
| Log10 HIV-1 RNA | 4.78 (1.02) | 4.71 (0.98) | |
| Ascaris lumbricoides | n=26 | n=28 | |
| CD4 count (cells/mm3) | 504.1 (238.3) | 561.6 (298.2) | |
| Log10 HIV-1 RNA | 4.89 (0.95) | 4.68 (0.80) | |
| Hookworm species | n=76 | n=72 | |
| CD4 count (cells/mm3) | 540.4 (240.9) | 584.0 (302.7) | |
| Log10 HIV-1 RNA | 4.79 (1.04) | 4.85 (0.99) | |
| Trichuris trichiura | n=12 | n=12 | |
| CD4 count (cells/mm3) | 619.8 (304.7) | 694.8 (394.3) | |
| Log10 HIV-1 RNA | 4.34 (0.85) | 4.37 (1.23) | |
| Mixed infections | n=7 | n=12 | |
| CD4 count (cells/mm3) | 587.7 (288.5) | 672.0 (407.4) | |
| Log10 HIV-1 RNA | 4.72 (0.86) | 5.13 (0.95) | |
Helminth infection
Of the 208 individuals included in the analysis, 148 were infected with hookworm species (71.2%), 54 (26.0%) were infected with A.lumbricoides and 24 (11.5%) with T. trichuri. Nineteen individuals (9.1%) were infected with more than one soil-transmitted helminth species. Almost all infections (96.2%) were classified as ‘light’ burdens based on WHO guidelines[9]. Two individuals with hookworm infection (1.4%) had moderate and two (1.4%) had heavy burdens of infection. There were 3 moderate A. lumbricoides infections (5.6%) and 1 moderate T. trichuria infection (4.2%). No individuals with A. lumbricoides or T. tichiura infection had heavy infections. All of the six individuals with moderate burden and both individuals with heavy burden of infection were infected with more than one helminth species.
Effect of treatment
CD4 counts
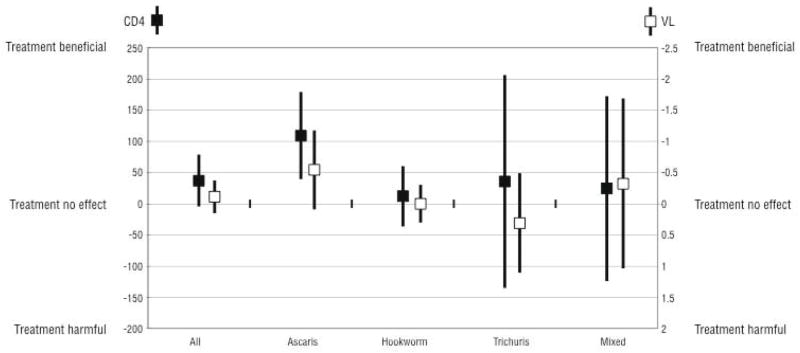
Treatment with albendazole was associated with a trend for higher CD4 counts at the 12 week follow up visit when compared to placebo (+37 cells/mm3, 95% CI −4.6 to +78.7 cells/mm3), (p=0.08) (Table 3). There was a highly significant effect of treatment among individuals with A. lumbricoides co-infection: individuals with ascariasis who received albendazole had a mean CD4 count at follow up that was 109 cells/mm3 higher than those who received placebo (95% CI +38.9 to +179.0 cells/mm3) (p=0.003, Table 3). There were no significant differences between the treatment arms in follow-up CD4 count among those infected with hookworm, T. trichiura, or those with mixed infection. Albendazole treatment was significantly more beneficial among individuals with A. lumbricoides co-infection than among those with other helminth species (p=0.04), (Figure 2).
Table 3.
Difference in CD4 counts and HIV-1 RNA levels in treatment arm versus placebo arm.
| Average difference in CD4 count between albendazole and placebo groups at 12 weeks follow-up | (95%CI) | P value | Average difference in Log10 HIV-1 RNA between albendazole and placebo groups at 12 weeks follow-up | (95%CI) | P value | |
|---|---|---|---|---|---|---|
|
All ITT individuals (n=208) |
+37.0 cells/mm3 | (−4.6 to +78.7) | 0.08 | −0.11 log10 copies/mL | (−0.37 to +0.15) | 0.42 |
|
Ascaris lumbricoides (n=54) |
+109.0 cells/mm3 | (+38.9 to +179.0) | 0.003 | −0.54 log10 copies/mL | (−1.17 to +0.09) | 0.09 |
|
Hookworm (n=148) |
+11.9 cells/mm3 | (−36.6 to 60.3) | 0.63 | +0.001 log10 copies/mL | (−0.30 to +0.30) | 1.00 |
|
Trichuris trichiura (n=24) |
+35.6 cells/mm3 | (−135.3 to +206.5) | 0.67 | +0.31 log10 copies/mL | (−0.49 to +1.10) | 0.43 |
|
Mixed Infection (n=19) |
+24.2 cells/mm3 | (−124.0 to +172.5) | 0.73 | −0.32 log10 copies/mL | (−1.68 to +1.03) | 0.62 |
Figure 2.
Differences in 12 week Follow-up CD4 counts and Viral Load
Plasma HIV-1 RNA levels
Among the 208 individuals included in the intent to treat analysis, there were no significant differences in log10 plasma HIV-1 RNA levels between the treatment groups, (p=0.42) (Table 3). There were no significant differences in the 12 week follow up log10 plasma HIV-1 RNA levels between the treatment groups when stratified by the other infecting helminth species (Figure 2). There was a trend for decrease in plasma HIV-1 RNA among individuals with A. lumbricoides co-infection who were treated with albendazole (−0.54 log10 plasma HIV-1 RNA, 95% CI −1.17 to 0.09 log10 copies/mL) (p=0.09) (Table 3).
Helminth Infection
Stool was provided for repeat analysis by 88 participants in the albendazole arm (81.5%) and by 80 participants in the placebo arm (80%) at the twelve week follow up visit (p=0.79). Individuals in the placebo arm were significantly more likely to have evidence of helminth infection at the twelve week follow up visit than those in the albendazole arm (40.0% vs. 21.6%, p=0.01). Of eight individuals with moderate or heavy burdens of infection at baseline and who provided stool for analysis at the follow up visit, three had been randomized to albendazole and five had been randomized to placebo. None of the three individuals in the albendazole group had evidence of infection at the 12 week visit while two of those in the placebo group had detectable helminth infection at the follow up visit.
Adverse Events
There were no adverse events reported during the course of this study.
Discussion
This study is the first randomized placebo-controlled study to determine effects of eradication of soil-transmitted helminths on markers of HIV-1 progression. It provides compelling evidence to suggest significant CD4 benefit of albendazole in A. lumbricoides co-infected individuals. Previous observational studies on soil transmitted helminth infection and HIV-1 have yielded inconsistent results and were limited in ability to control for confounding effects because of their observational design[12–16]. This study yields several important findings. First, a 3-day course of albendazole resulted in significantly higher CD4 counts in A. lumbricoides infected individuals when compared to placebo. The direction of effect on plasma HIV-1 RNA levels in the A. lumbricoides infected individuals was consistent with the CD4 findings. Benefits of albendazole were seen despite overall light intensities of helminth infection and despite the detection of helminths in some albendazole recipients following treatment, suggesting persistence or reinfection.
Our data provide evidence to support previous models suggesting that immune modulation due to helminth infection may affect HIV-1 progression, including effects on both CD4 counts and plasma HIV-1 RNA viral load in co-infected individuals[17, 18]. Helminth infection leads to significant immune activation, which results in increased HIV-1 replication in both blood and lymphoid tissue and is a key correlate of HIV-1 disease progression[2, 17, 19–26]. In addition, chronic helminth infection is characterized by a dominant Th2 immune profile with subsequent reductions in HIV-1 specific cellular immune responses, which may decrease immune control of HIV-1 replication[17, 27–30]. A. lumbricoides is significantly larger than other intestinal helminths and induces a more highly skewed Th2 response than do other helminth species[31–35]. In this clinical trial, treatment of A. lumbricoides co-infection resulted in significantly higher CD4 counts than placebo after 12 weeks of follow up, perhaps suggesting that decreased CTL-responses due to helminth infection may contribute to the observed effect of A. lumbricoides treatment on CD4 count. The differences in effect between helminth species observed in this study are consistent with previous reports of species-specific differences in observational HIV-1 infected helminth infected cohorts, specifically with treatment of A. lumbricoides and Mansonella species[12, 14, 16]. Further evaluations of the effects of individual species of helminths on markers of immune activation and HIV-1 specific immune responses are needed to clarify the mechanisms underlying this observation.
The principal strengths of this study were its randomized, double-blind, placebo-controlled design and the inclusion of multiple, geographically diverse sites in Kenya. The study used robust biologic markers of HIV-1 progression (CD4 counts and plasma HIV-1 RNA). Despite these strengths, there were several potential limitations of this study. Most individuals enrolled in this study (>96%) had low burdens of helminth infection based on WHO criteria. Intensity of helminth infection has been correlated with HIV-1 viral load[16]. Thus, it is possible that the low intensity of helminth infections resulted in less likelihood of detecting benefit from helminth eradication. The study was also limited by a relatively short duration of follow-up (3 months). However, deferring treatment of helminth-infected individuals for a longer time period may not be acceptable to participants.
Although the low worm burden in the cohort may have decreased the likelihood of detecting a treatment effect, the intensity of helminth infection in this cohort reflects the population dispersion of helminth infection among adults in Africa. In contrast to children, adults are typically infected with low worm burdens with most helminth species other than hookworm[36]. Demonstrating an effect in this low-intensity population suggests a more generalizable effect than a trial restricted to individuals with high worm burden. Low worm burden in the cohort is further evidenced by our observation that only 40% of individuals receiving placebo in this cohort had detectable helminth ova at follow up, despite all of these individuals having detectable helminths at enrolment. This underscores the limitations of stool screening, which has low sensitivity for detection of low burdens of helminth infection[37]. It is therefore likely that our initial screening failed to detect individuals with low helminth burden who may have benefited from treatment. In addition, some of the individuals included in the study with hookworm or T. trichiura may have also harbored undetected A. lumbricoides infection. Empiric deworming of all HIV-1 infected individuals residing in helminth-endemic regions without stool screening is therefore an alternative strategy that deserves further study.
Our data suggest that treatment of helminth co-infection may be a practical and cost-effective intervention to delay disease progression in HIV-1 infected individuals in resource-limited settings. Studies conducted in Africa have estimated the average rate of CD4 decline at between 20 and 30 cells/mm3 per year[38, 39]. An increase in CD4 count as seen with treatment of A. lumbricoides in this study could potentially prolong the time to severe immunosuppression and need for antiretroviral medication by several years. A trend towards a 0.54 log10 copies/mL reduction in plasma HIV-1 RNA was seen with treatment of A. lumbricoides in this study. Modeling of the effect of a vaccine that reduced HIV-1 RNA suggests that a reduction in viral load of this magnitude would delay progression to AIDS by 3.5 years and slow the need for antiretroviral medications by almost a full year[40]. Current estimates suggest that as many as one and a half billion people are currently infected with A. lumbricoides, predominantly in areas of the world with substantial burdens of HIV-1 infection[41]. The public health and economic implications of such benefits are potentially enormous and warrant further investigation.
Other bacterial, viral and parasitic co-infections may have unique effects on host-immune and immune-HIV interactions and interventions to treat or prevent these infections may alter HIV-1 progression by different mechanisms. As evidence for the benefits of treating these various co-infections emerges, the role of combined interventions should also be assessed.
We have shown that treatment of A. lumbricoides is associated with significant improvements in CD4 counts and may potentially reduce plasma HIV-1 RNA viral load. Further clinical trials are needed to evaluate the long-term durability of the response to deworming in HIV-1 co-infected individuals and to assess whether empiric therapy of all HIV-1 infected individuals in helminth endemic areas is warranted.
Acknowledgments
We would like to thank all of the participants and the clinics and organizations caring for persons living with HIV/AIDS who participated in this study; the staff of the University of Washington/KEMRI/FHCRC; Ben Piper, Dr. Frederick Kirui, Jonathan Chebotibin, Beryl Obura, Loice Wangari Mbogo, Andele Nyambura, Benendine Bukachi, Josephine Gichuhi; Sandy Emery, Alun Davies, Professor Zvi Bentwich, Dr. Cameron Page, Dr. Monique Wasunna, Dr. Kevin Marsh, Dr. Jack Nyamongo, Dr. Ernest Makhoha; Glaxo-Smith-Kline who provided all study medication and placebo, Alpha-Tec, USA who provided all stool collection containers. This paper was published with permission of the Director of the Kenya Medical Research Institute (KEMRI).
This research was supported by the Royalty Research Fund at the University of Washington and the US National Institutes of Health (NIH) research grant, CFAR SUPP OAR FWA00006878. All active drug (albendazole) and placebo were provided at no cost by Glaxo-Smith-Kline. All stool collection containers were provided by Alpha-Tec, USA.
Footnotes
The authors have no conflict of interest with any commercial or other association in conjunction with the research presented herein.
Author Justification
Judd L. Walson, MD, MPH
I, Judd L Walson, declare that I participated in the above-mentioned study as the lead investigator. I was involved in the development of the proposal, implementation of the study, data collection and preparation of the manuscript. I have seen and have approved the final version that is being submitted for publication. I have no conflicts of interest.
Phelgona A. Otieno MBChB, MMed, MPH
I, Phelgona Apondi Otieno declare that I participated in the above-mentioned study as a Co-principal Investigator. I was involved in the development of the proposal, submission for local ethical clearance, recruitment of study staff, data collection and review of the manuscript. I have seen and have approved the final version that is being submitted for publication. I have no conflicts of interest.
Margaret Mbuchi, PhD
I, Margaret Mbuchi, declare that I participated in the design of the study, implementation ond participant recruitment and collection of data, and that I have seen and approved the final version. I have no conflicts of interest.
Barbra A. Richardson, PhD
I, Barbra Richardson, declare that I participated in the design of study, analysis of data, and interpretation of results and that I have seen and approved the final version. I have the following conflicts of interest: none.
Barbara Lohman-Payne, PhD
I, Barbara Lohman-Payne, declare that I participated in the study design as well as the collection and interpretation of data. I have seen and approved the final version. I have no conflicts of interest.
Steve Wanyee Macharia MS
I, Steve Wanyee Macharia, declare that I participated in the study design as well as the collection and management of data. I have seen and approved the final version. I have no conflicts of interest.
Julie Overbaugh PhD
I, Julie Overbaugh, declare that I participated in the implementation of the study and the data analysis. I also contributed to the interpretation of results. I have seen and approved the final version. I have the following conflicts of interest: none.
James Berkley MB BS MTropMed MRCP MD
I, James Berkley, declare that I participated study design and implementation. I also contributed to the data analysis and preparation of the final manuscript. I have seen and have approved the final version that is being submitted for publication. I have no conflicts of interest.
Eduard J. Sanders, MD, PhD
I, Eduard Sanders, declare that I participated in the above-mentioned study. I was involved in the implementation of the study at a site and in review of the manuscript. I have seen and have approved the final version that is being submitted for publication. I have no conflicts of interest.
Michael Chung, MD, MPH
I, Michael Chung, declare that I participated in the above-mentioned study. I was involved in the implementation of the study at a site and in review of the manuscript. I have seen and have approved the final version that is being submitted for publication. I have no conflicts of interest.
Grace C. John-Stewart MD, PhD
I, Grace John-Stewart, declare that I participated in designing the study, obtaining funding, and provided input on analyses and writing the manuscript and that I have seen and approved the final version. I have no conflicts of interest
References
- 1.World Health Organization. Weekly epidemiological record. 2006. Schistosomiasis and soil-transmitted helminth infections - preliminary estimates of the number of children treated with albendazole or mebendazole; pp. 145–164. [PubMed] [Google Scholar]
- 2.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 4.Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003;86:315–333. doi: 10.1016/s0001-706x(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 5.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. 2005;192:1956–1961. doi: 10.1086/497696. [DOI] [PubMed] [Google Scholar]
- 6.Walson JLJ-SG. Treatment of helminth co-infection in HIV-1 infected individuals in resource-limited settings. Cochrane Database of Systematic Reviews. 2008:1. doi: 10.1002/14651858.CD006419.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 8.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins KRQ-MM, Toossi Z, Arts EJ. Impact of tuberculosis on HIV-1 replication, diversity and disease progression. AIDS Rev. 2002;4:165–176. [PubMed] [Google Scholar]
- 10.Otieno PA, Brown ER, Mbori-Ngacha DA, Nduati RW, Farquhar C, Obimbo EM, et al. HIV-1 disease progression in breast-feeding and formula-feeding mothers: a prospective 2-year comparison of T cell subsets, HIV-1 RNA levels, and mortality. J Infect Dis. 2007;195:220–229. doi: 10.1086/510245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers AJAD. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown M, Kizza M, Watera C, Quigley MA, Rowland S, Hughes P, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis. 2004;190:1869–1879. doi: 10.1086/425042. [DOI] [PubMed] [Google Scholar]
- 13.Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. 2003;97:103–108. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- 14.Modjarrad K, Zulu I, Redden DT, Njobvu L, Lane HC, Bentwich Z, Vermund SH. Treatment of intestinal helminths does not reduce plasma concentrations of HIV-1 RNA in coinfected Zambian adults. J Infect Dis. 2005;192:1277–1283. doi: 10.1086/444543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwanakasale VVP, Sukwa TY, Ziba M, Ernest A, Tanner M. Interactions between schistosoma haematobium and human immunodeficiency virus type 1: The effects of coinfection on treatment outcomes in rural Zambia. American Journal of Tropical Medicine and Hygiene. 2003;69:420–428. [PubMed] [Google Scholar]
- 16.Wolday D, Mayaan S, Mariam ZG, Berhe N, Seboxa T, Britton S, et al. Treatment of intestinal worms is associated with decreased HIV plasma viral load. J Acquir Immune Defic Syndr. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Borkow G, Bentwich Z. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 2006;28:605–612. doi: 10.1111/j.1365-3024.2006.00918.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown M, Mawa PA, Kaleebu P, Elliott AM. Helminths and HIV infection: epidemiological observations on immunological hypotheses. Parasite Immunol. 2006;28:613–623. doi: 10.1111/j.1365-3024.2006.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M, Bentwich Z. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–421. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 21.Kassu A, Tsegaye A, Wolday D, Petros B, Aklilu M, Sanders EJ, et al. Role of incidental and/or cured intestinal parasitic infections on profile of CD4+ and CD8+ T cell subsets and activation status in HIV-1 infected and uninfected adult Ethiopians. Clin Exp Immunol. 2003;132:113–119. doi: 10.1046/j.1365-2249.2003.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lederman MM, Kalish LA, Asmuth D, Fiebig E, Mileno M, Busch MP. ‘Modeling’ relationships among HIV-1 replication, immune activation and CD4+ T-cell losses using adjusted correlative analyses. Aids. 2000;14:951–958. doi: 10.1097/00002030-200005260-00006. [DOI] [PubMed] [Google Scholar]
- 23.Leng Q, Bentwich Z, Magen E, Kalinkovich A, Borkow G. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. Aids. 2002;16:519–529. doi: 10.1097/00002030-200203080-00002. [DOI] [PubMed] [Google Scholar]
- 24.Leng Q, Borkow G, Weisman Z, Stein M, Kalinkovich A, Bentwich Z. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27:389–397. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Kamal SM, El Sayed Khalifa K. Immune modulation by helminthic infections: worms and viral infections. Parasite Immunol. 2006;28:483–496. doi: 10.1111/j.1365-3024.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001;14:753–777. doi: 10.1128/CMR.14.4.753-777.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci U S A. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalod M, Dupuis M, Deschemin JC, Sicard D, Salmon D, Delfraissy JF, et al. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McElroy MDEM, Jones N, Ssali F, Mugyenyi P, Barugahare B, et al. Coinfection with schistosoma mansoni is associated with decreased HIV-specific cytolysis and increased IL-10 production. The Journal of Immunology. 2005;174:5119–5123. doi: 10.4049/jimmunol.174.8.5119. [DOI] [PubMed] [Google Scholar]
- 30.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 31.Bradley JE, Jackson JA. Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunol. 2004;26:429–441. doi: 10.1111/j.0141-9838.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- 32.Cooper PJ, Chico ME, Sandoval C, Espinel I, Guevara A, Kennedy MW, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–1213. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 33.Geiger SM, Caldas IR, Mc Glone BE, Campi-Azevedo AC, De Oliveira LM, Brooker S, et al. Stage-specific immune responses in human Necator americanus infection. Parasite Immunol. 2007;29:347–358. doi: 10.1111/j.1365-3024.2007.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson JA, Turner JD, Rentoul L, Faulkner H, Behnke JM, Hoyle M, et al. Cytokine response profiles predict species-specific infection patterns in human GI nematodes. Int J Parasitol. 2004;34:1237–1244. doi: 10.1016/j.ijpara.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Pit DS, Polderman AM, Baeta S, Schulz-Key H, Soboslay PT. Parasite-specific antibody and cellular immune responses in human infected with Necator americanus and Oesophagostomum bifurcum. Parasitol Res. 2001;87:722–729. doi: 10.1007/s004360100419. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. WHO technical report series. 2001. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. [PubMed] [Google Scholar]
- 37.Brown MBJ, Hughes P, Nakiyingi J, Watera C, Elliott A, Whitworth J. Screening for intestinal helminth infestation in a semi-urban cohort of HIV-infected people in Uganda: a combination of techniques may enhance diagnostic yield in the absence of multiple stool samples. Tropical Doctor. 2003;33:72–76. doi: 10.1177/004947550303300206. [DOI] [PubMed] [Google Scholar]
- 38.Katubulushi M, Zulu I, Yavwa F, Kelly P. Slow decline in CD4 cell count in a cohort of HIV-infected adults living in Lusaka, Zambia. Aids. 2005;19:102–103. doi: 10.1097/00002030-200501030-00016. [DOI] [PubMed] [Google Scholar]
- 39.Urassa W, Bakari M, Sandstrom E, Swai A, Pallangyo K, Mbena E, et al. Rate of decline of absolute number and percentage of CD4 T lymphocytes among HIV-1-infected adults in Dar es Salaam, Tanzania. Aids. 2004;18:433–438. doi: 10.1097/00002030-200402200-00009. [DOI] [PubMed] [Google Scholar]
- 40.Gupta SB, Jacobson LP, Margolick JB, Rinaldo CR, Phair JP, Jamieson BD, et al. Estimating the benefit of an HIV-1 vaccine that reduces viral load set point. J Infect Dis. 2007;195:546–550. doi: 10.1086/510909. [DOI] [PubMed] [Google Scholar]
- 41.Chan MS. The global burden of intestinal nematode infections--fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]