Abstract
1. The property of insect fibrillar muscle which enables it to oscillate continuously when it is connected to a resonant load is the delayed activation by stretch of contractile activity. The dynamic response has been measured at different fibre lengths and at different calcium ion concentrations, to see what effects these conditions have on the magnitude and rate constant of the delayed tension.
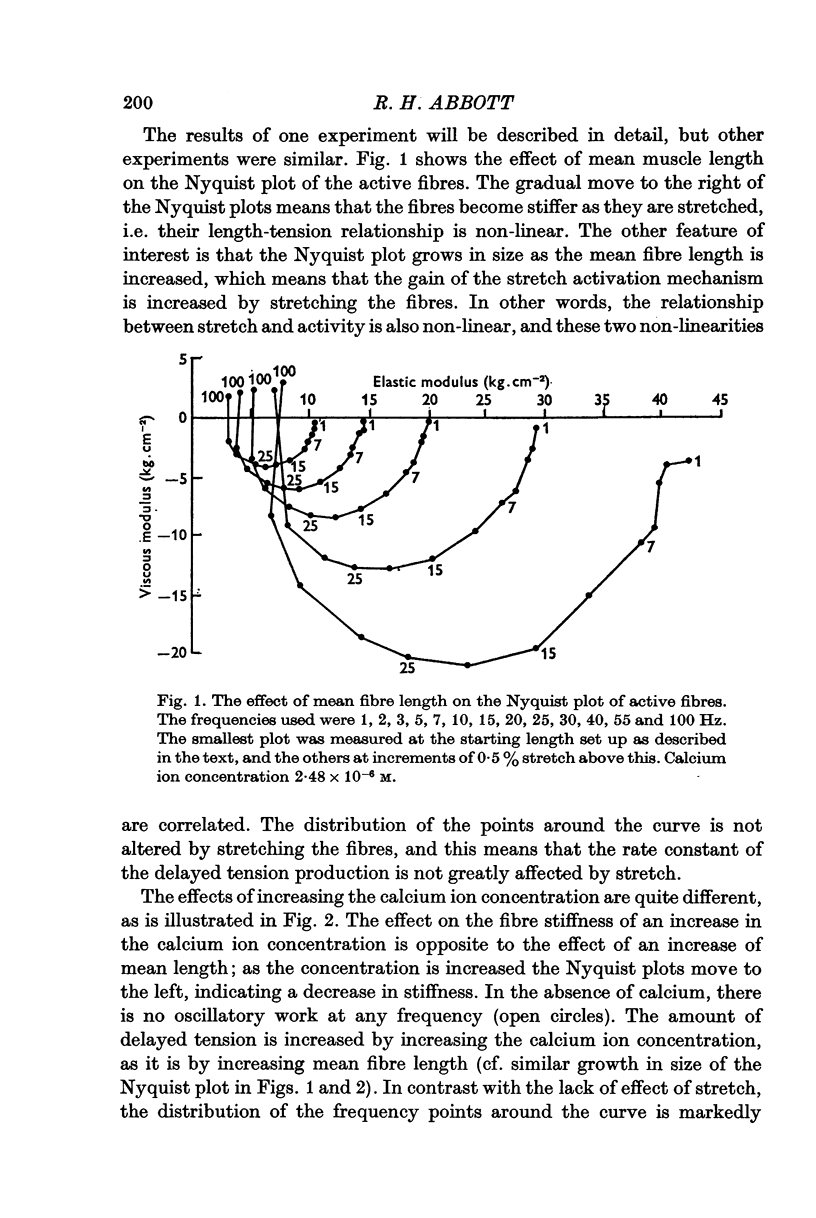
2. Bundles of ten fibres of the dorsal longitudinal muscle of the water bug, Lethocerus cordofanus, which had been in glycerol for less than 5 days were used. Graded activation was obtained with buffered calcium ion concentrations between 10-7 and 10-5 M.
3. A computer-controlled apparatus was used to measure the dynamic mechanical properties of the muscle fibres. This allowed many measurements to be made on the same preparation. Further computer programs analysed the Nyquist plots produced by the experiment.
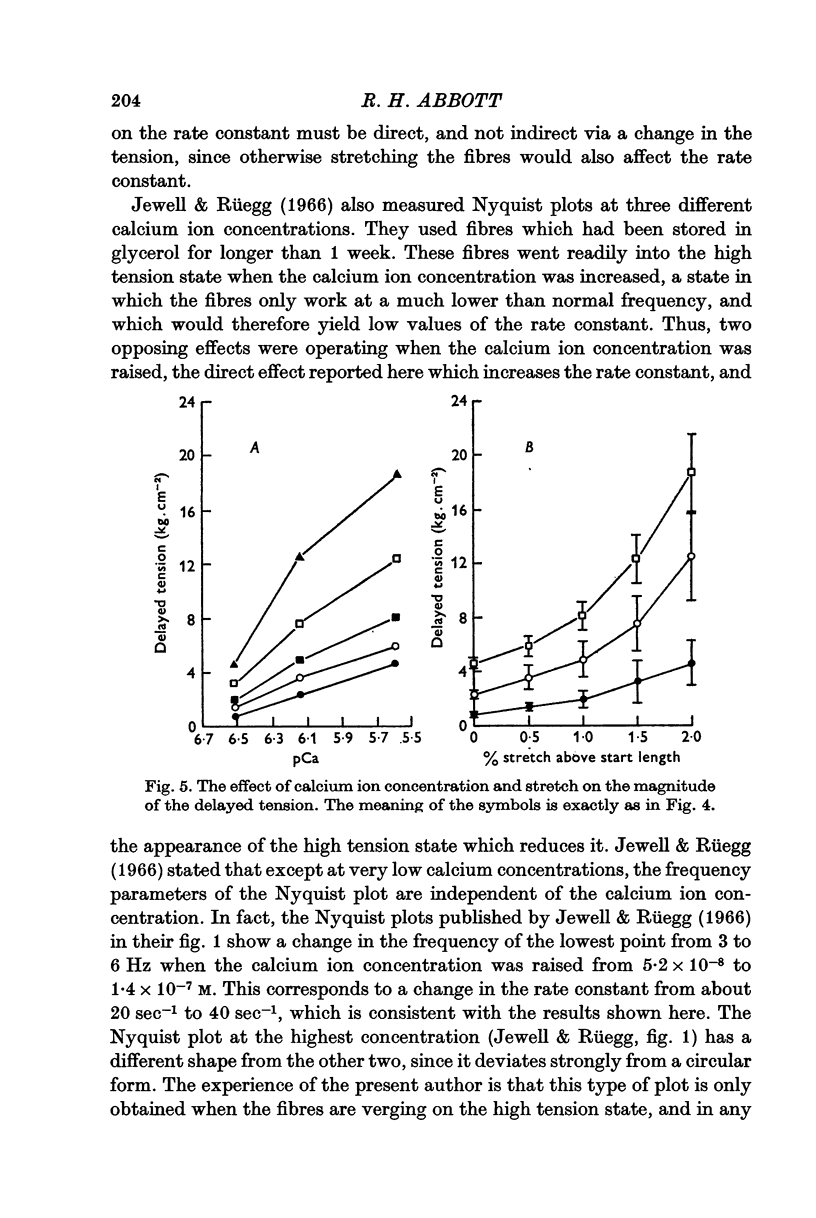
4. When the mean length of a fibre bundle was increased, the magnitude of the delayed tension was increased (for a constant amplitude of sinusoidal length change), the rate constant was unaltered, and the stiffness of the fibres was increased. When the calcium ion concentration was raised, the magnitude of the delayed tension was increased, the rate constant increased, and the stiffness of the fibres fell. Calcium activation and stretch activation are thus clearly separable in their effects, and so the mechanisms must be separate.
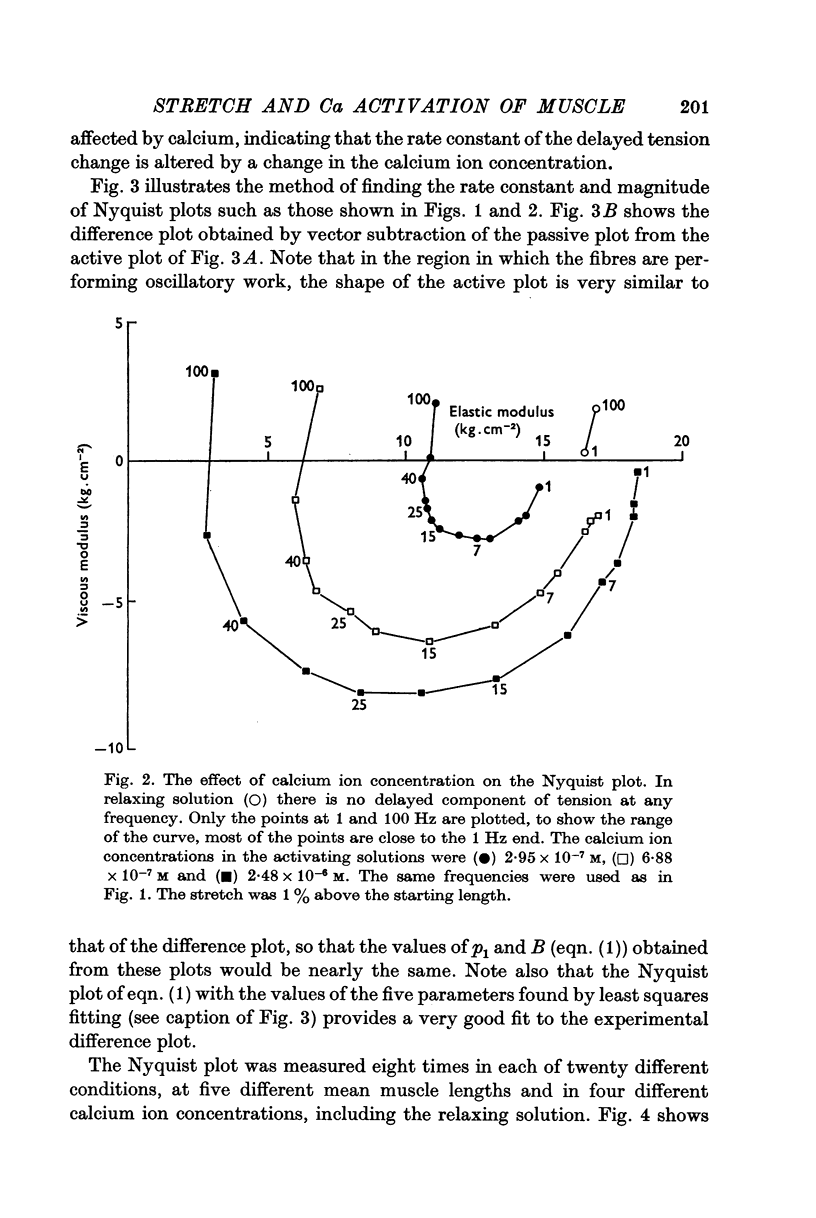
5. The various different effects of calcium cannot be explained by any simple model of activation, for example, an on-off switch mechanism controlling the number of bridges in action.
6. The stretch-induced activity is proportional to a power of the length of two or greater and this non-linearity aids the efficient operation of the oscillatory mechanism.
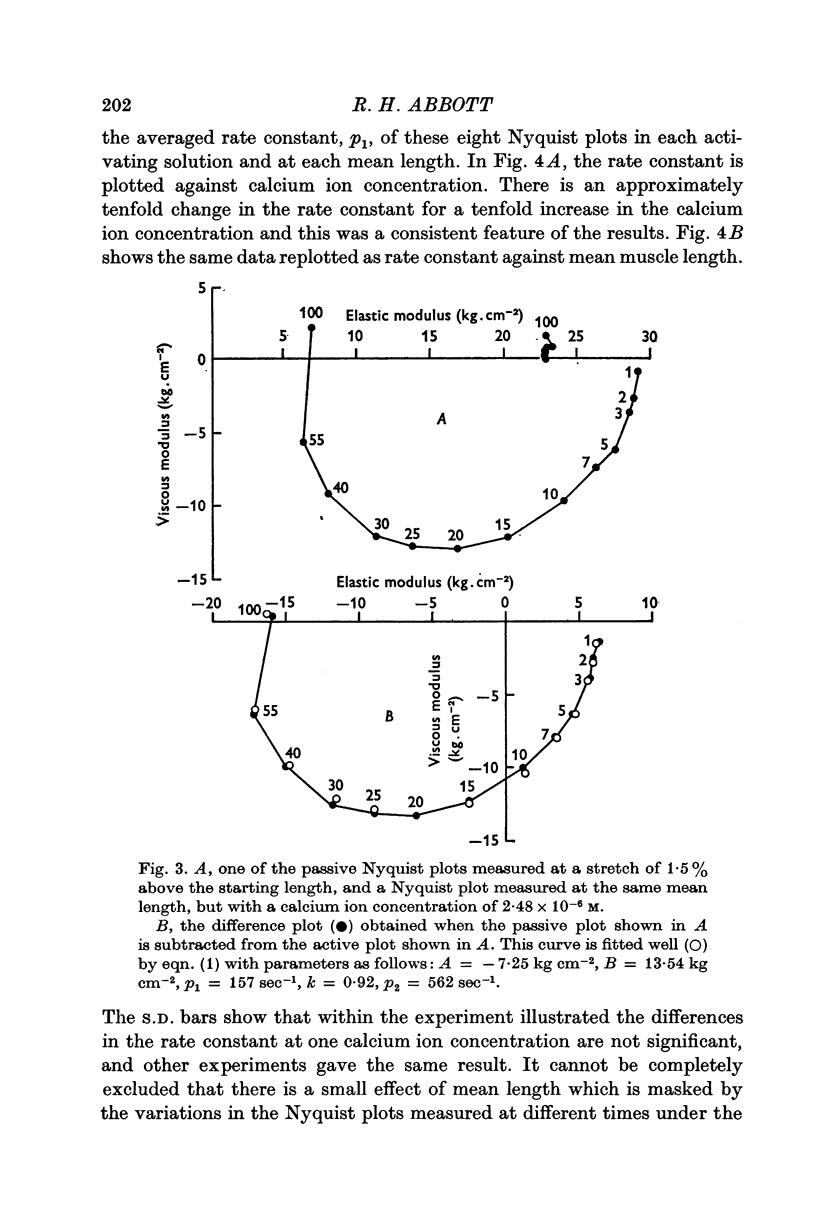
Full text
PDF




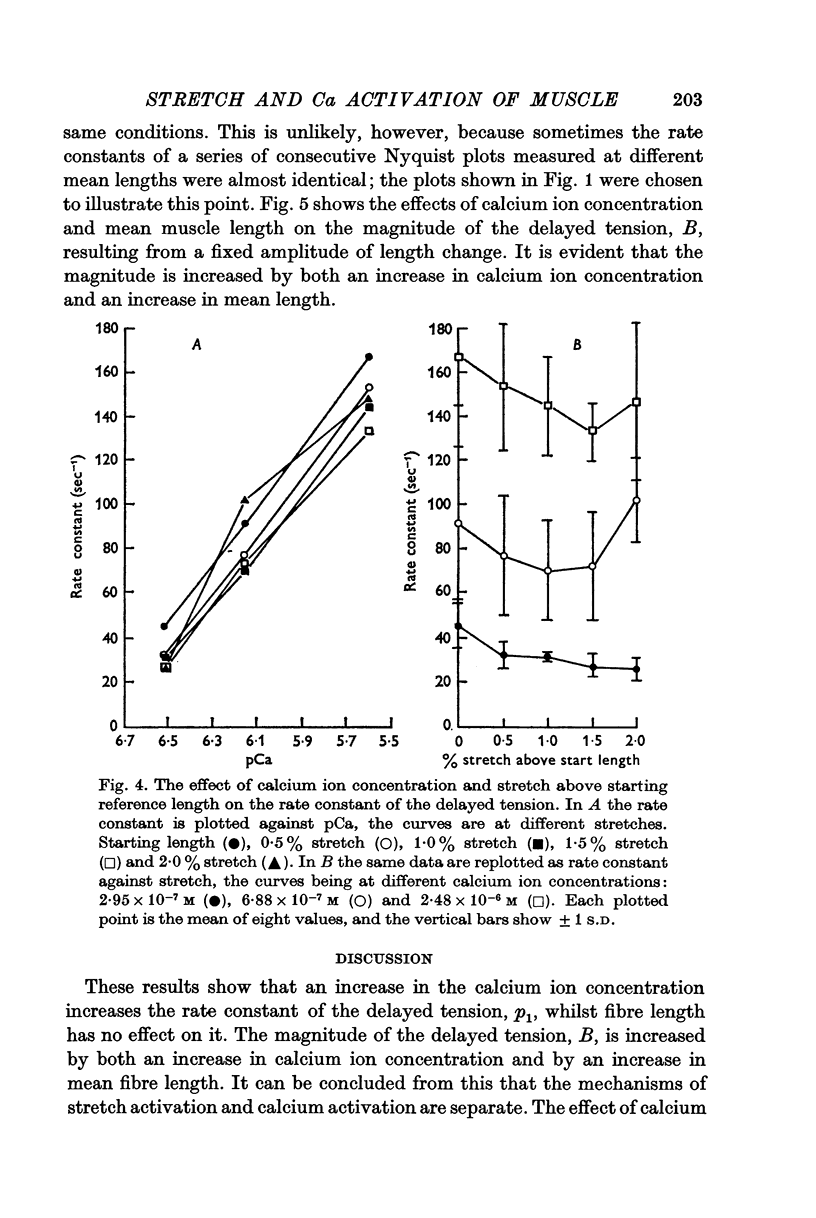




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Chaplain R. A. Preparation and properties of the contractile element of insect fibrillar muscle. J Cell Sci. 1966 Sep;1(3):311–330. doi: 10.1242/jcs.1.3.311. [DOI] [PubMed] [Google Scholar]
- Abbott R. H. Computer control of mechanical experiments on muscle. Biochem J. 1971 Jan;121(1):3P–4P. doi: 10.1042/bj1210003pc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott R. H., Mannherz G. H. Activation by ADP and the correlation between tension and ATPase activity in insect fibrillar muscle. Pflugers Arch. 1970;321(3):223–232. doi: 10.1007/BF00588443. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHIN K. E., PRINGLE J. W. The physiology of insect fibrillar muscle. III. The effect of sinusoidal changes of length on a beetle flight muscle. Proc R Soc Lond B Biol Sci. 1960 Jun 14;152:311–330. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Perrin D. D., Sayce I. G. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967 Jul;14(7):833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg J. C., Tregear R. T. Mechanical factors affecting the ATPase activity of glycerol-extracted insect fibrillar flight muscle. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):497–512. doi: 10.1098/rspb.1966.0080. [DOI] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]



