Abstract
UVA (320-400 nm) radiation constitutes >90% of the environmentally relevant solar UV radiation, and it has been proposed to have a role in skin cancer and aging. Because of the popularity of UVA tanning beds and prolonged periods of sunbathing, the potential deleterious effect of UVA has emerged as a source of concern for public health. Although generally accepted, the impact of DNA damage on the cytotoxic, mutagenic, and carcinogenic effect of UVA radiation remains unclear. In the present study, we investigated the sensitivity of a panel of yeast mutants affected in the processing of DNA damage to the lethal and mutagenic effect of UVA radiation. The data show that none of the major DNA repair pathways, such as base excision repair, nucleotide excision repair, homologous recombination, and postreplication repair, efficiently protect yeast from the lethal action of UVA radiation. In contrast, the results show that the Ogg1 DNA glycosylase efficiently prevents UVA-induced mutagenesis, suggesting the formation of oxidized guanine residues. Furthermore, sequence analysis of UVA-induced canavanine-resistant mutations reveals a bias in favor of GC→TA events when compared with spontaneous or H2O2-, UVC-, and γ-ray- induced canavanine-resistant mutations in the WT strain. Taken together, our data point out a major role of oxidative DNA damage, mostly 7,8-dihydro-8-oxoguanine, in the genotoxicity of UVA radiation in the yeast Saccharomyces cerevisiae. Therefore, the capacity of skin cells to repair 7,8-dihydro-8-oxoguanine may be a key parameter in the mutagenic and carcinogenic effect of UVA radiation in humans.
Keywords: base excision repair, OGG1, mutagenesis, DNA photolesions
UVA radiation has been proposed to have a role in skin cancer and aging (1). UVA (320-400 nm) constitutes >90% of the environmentally relevant solar UV radiation. Because of the popularity of the high-intensity UVA tanning equipment and the widespread use of efficient UVB-absorbing sunscreens blocking erythema and often accompanied by prolonged periods of sunbathing, the human exposure to UVA have increased significantly in the last decades. The deleterious effect of UVA has, as a consequence, recently emerged as a source of concern for public health (2, 3).
Although very complex, the biological effect of UVA radiation is at least partially related to DNA damage. Despite it being well established that UVA can damage DNA, to date, the nature of the lesions underlying its mutagenic and carcinogenic effects remains controversial. Unlike UVB photons, which are directly absorbed by DNA and cause the formation of cis-syn cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts, the UVA component of solar radiation is poorly absorbed by DNA. Rather, UVA radiation excites other endogenous chromophores, generating reactive oxygen species, some of which are possibly involved in the outcome of photocarcinogenesis (4, 5). It is generally accepted that UVA damages DNA, either through the generation of singlet oxygen (1O2) or by a type-1 photosensitization reaction resulting in oxidative base modifications, such as 7,8-dihydro-8-oxoguanine (8-oxoG) (6-10). However, more recently, it has been reported that UVA induces CPDs in rodent and human skin cells, predominantly at thymine-thymine sequences and at a higher rate than 8-oxoG (8, 11-14). As yet, the mutagenic role for UVA based on the production of 8-oxoG and on the formation of CPDs has been suggested. Indeed, Besaratinia et al. (15) observed a majority (25%) of GC→TA transversions in mouse embryonic fibroblasts, the expected mutation type for 8-oxoG. In contrast, this type of mutation is poorly represented in other studies (16-18), and the mutations found correlate with the formation of CPDs (13). In Chinese hamster ovary cells, the most prevalent UVA-induced mutational class was AT→CG transversion, whereas CG→TA transitions at dipyrimidine sites, the signature for CPDs, were also frequent (16). In human cells, this last class of mutational events predominated, although an increase in mutations at AT base pairs was observed in comparison with UVB (17). In human squamous skin tumors, the observation of AT→CG and GC→TA transversions in the basal layer has been interpreted as a role for UVA-induced DNA damage in human skin cancinogenesis (19).
All of the above studies have been performed with different UVA sources and conditions of exposure and with various cellular systems and target genes for mutation analysis, which makes comparison difficult. In the present study, we took advantage of the availability of yeast Saccharomyces cerevisiae strains mutated in different DNA repair genes to exhaustively investigate the cytotoxic and mutagenic effects of UVA radiation. Initially, we focused on the base excision repair (BER) pathway, which is responsible for the elimination of the major part of oxidative DNA damages. We used cells mutated in genes coding for DNA glycosylases (Ogg1p, Ntg1p, and Ntg2p) involved in the removal of oxidatively damaged DNA bases and cells mutated in genes coding for abasic (AP) site endonucleases (Apn1p and Apn2p) (20, 21). The role of other repair pathways on UVA-induced cytotoxicity and mutagenesis was also explored, i.e., nucleotide excision repair (NER) mediated by the Rad14p, homologous recombination (HR) mediated by the Rad51p, postreplication repair mediated by the Rad18p, and translesion DNA synthesis (TLS) by specialized DNA polymerases, such as Rad30p and Rev3p (22, 23). The biological impact of UVA radiation was also investigated by using strains that are affected in multiple DNA repair pathways. Therefore, the yeast strains used in this study provide very sensitive tools to identify cytotoxic and mutagenic DNA lesions after exposure to UVA radiation. The formation of 8-oxoG and CPDs was also determined by alkaline gel electrophoresis of DNA isolated from UVA-irradiated cells. Furthermore, UVA-induced mutation spectra were determined in WT, ogg1, and rad30 mutants by using a forward mutation assay with the CAN1 gene as a target. Our data strongly suggest that the cytotoxic action of UVA radiation is primarily independent of DNA repair. On the other hand, our findings point out a major role of oxidative DNA damage, mostly 8-oxoG, in the mutagenic effect of UVA radiation in S. cerevisiae.
Materials and Methods
Media and Growth Conditions. Yeast strains were grown in yeast extract/peptone/dextrose (YPD) medium (1% yeast extract/1% bactopeptone/2% glucose/2% agar for plates) or yeast nitrogen base and dextrose (YNBD) medium (0.17% yeast nitrogen base without amino acids/2% glucose/2% agar for plates) supplemented with appropriate amino acids and bases. All media, including agar, were purchased from Difco. Supplemented YNBD medium lacking arginine but containing canavanine (Sigma) at 60 mg/liter was used for the selective growth of canavanine-resistant (CanR) mutants.
Yeast Strains, Plasmids, and Microbiological Methods. S. cerevisiae strains used in the present study are listed in Table 1. Gene disruption was generated according to refs. 24 and 25. Disruptions were confirmed by PCR on genomic DNA. Plasmid pYSB210 contains the OGG1 gene placed under the control of the TPI promoter in the pYX212 plasmid (our laboratory stock).
Table 1. S. cerevisiae strains used in this study.
| Strain | Genotype | Function of mutated genes |
|---|---|---|
| FF18733 | MATa leu2-3-112 trp 1-289 his7-2 ura3-52 lys1-1 | — |
| FF18734 | MATα leu2-3-112 trp 1-289 his7-2 ura3-52 lys1-1 | — |
| FF181134 | FF18733 with Δrev3::URA3 | TLS |
| CD 138 | FF18733 with Δogg 1::TRP1 | BER |
| BG34 | FF18733 with Δrad14::LEU2 | NER |
| BG40 | FF18733 with Δrad14::LEU2 Δapn1::URA3 Δapn2::kanMX6 | NER, BER |
| BG206 | FF18734 with Δrad14::LEU2 Δrad51::LEU2 | NER, HR |
| BG310 | FF18733 with Δntg1::URA3 Δntg2::TRP1 Δrad14::kanMX6 | BER, NER |
| BG40 | FF18733 with Δrad14::URA3 Δogg1::TRP1 | NER, BER |
| BP300 | FF18733 with Δrad30::kanMX6 | TLS |
| BP3000 | FF18733 with Δogg1::TRP1 Δrad30::kanMX6 | BER, TLS |
| BPS1002 | FF18733 with Δrad18::kanMX6 | PRR |
| BPS1005 | FF18733 with Δogg1::TRP1 Δrev3::URA3 | BER, TLS |
| DGD39 | FF18733 with Δung1 Δntg1 Δntg2::kanMX6 Δogg1::URA3 Δmag1::hphMX4 | BER |
| SK-1 | FF18733 with Δphr1::kanMX6 | PHR |
| SK-2 | FF18733 with Δrad14::LEU2 Δphr1::kanMX6 | NER, PHR |
PRR, post-replication repair; PHR, photoreactivation repair.—, not applicable.
Sensitivity of Strains to UVA, UVC, H2O2, and γ-Radiation. Yeast strains were grown in 2 ml of YPD at 30°C to an OD600 = 1.0. Cells were harvested by centrifugation, washed twice with water, resuspended in 2 ml of sterile water, and then exposed to increasing doses of DNA-damaging agents. For UVA irradiation, cells were irradiated at a fluence rate of 80 mW/cm2 with a 5,000-W Supersun lamp (Mutzhas, Münich, Germany) emitting virtually exclusively in the 320- to 440-nm, UVA range (the energy at 315 nm is 5 × 10-6 times that at 365 nm; Fig. 6, which is published as supporting information on the PNAS web site). Cells were irradiated with 254-nm UVC at a dose rate of 0.12 mW/cm2. In the case of γ-irradiation, cells were exposed to a 137Cs source at a dose rate of 52.4 Gy/min. Cells were exposed to H2O2 (Sigma) for 30 min at 30°C under shaking. For all experiments, untreated and treated cells were immediately diluted and plated on YPD agar. Colonies were scored after 3 days of growth at 30°C. All experiments were carried out independently at least three times.
Induced Mutation Frequencies and Mutation Spectra. After treatment, yeast cells were harvested by centrifugation, washed twice with water and resuspended in 2 ml of YPD medium. Untreated and treated cultures were grown for an additional 48 h at 30°C. Cell density was measured by counting cells under a microscope or by plating dilutions on YPD or YNBD agar plates and counting the colonies after 3 days at 30°C. The quantification of CanR mutants was determined after plating on selective medium (YNBD containing canavanine). Colonies were counted after 3-4 days at 30°C. All experiments were carried out independently at least three times. Mutation frequencies were determined from the number of CanR colonies per milliliter divided by the number of viable cells per milliliter and expressed as averages. Spontaneous mutation frequencies were determined as described by using the method of the median (26, 27). CanR mutation spectra were determined as described in refs. 27 and 28 (details are available in Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
Detection of CPDs and 8-OxoG. Upon UVA irradiation, rad14 ogg1 double-mutant cells were harvested, and genomic DNA was immediately extracted in the presence of 0.1 mM desferrioxamine mesylate (Sigma) to prevent the formation of 8-oxoG during the procedure (29). Aliquots (10 μg) of DNA from irradiated or nonirradiated cells were incubated for 45 min at 37°C in a reaction buffer (12-μl final volume) that contained 250 ng of Escherichia coli Fpg protein (our laboratory stock) or 1,000 units of T4 denV protein (Trevigen, Gaithersburg, MD). The activity of the T4 denV protein was first calibrated on genomic DNA from UVC-irradiated yeast. Before loading on a 0.6% alkaline agarose gel, samples were denatured for 20 min at room temperature by adding 10 μl of 100 mM NaOH, 4 mM EDTA, and 10 μl of denaturing gel loading buffer (50% glycerol/1 M NaOH/0.2% bromocresol green). Electrophoresis was run overnight at 1.3 V/cm at room temperature.
Statistical Analysis. Statistical evaluation of differences in survival and mutation frequencies observed between strains according to UVA radiation dose was carried out by using a linear mixed model with random subject effect. All analysis were performed with s-plus6.2 statistical software (Insight, Seattle) (30). Mutation spectra were compared by using a two-tailed Fisher exact test.
Results
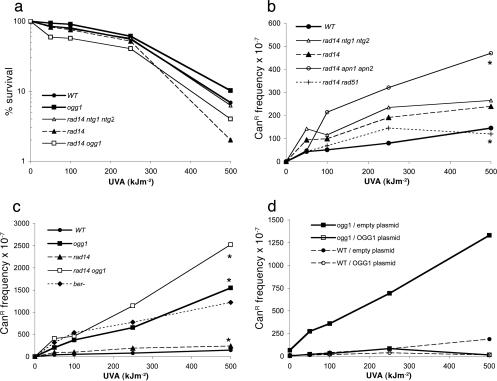
Sensitivity and Mutability of DNA Repair-Deficient Cells to UVA Radiation. To investigate the cytotoxic capacity of UVA in yeast, we used mutant strains of S. cerevisiae affected in DNA repair pathways, which are good indicators for the formation of lethal DNA lesions, such as alkylated bases, oxidized bases, AP sites, CPDs, and double-stranded breaks (DSBs) (22, 23). In our assay conditions, UVA exposure of WT cells causes cell death with a D37 of ≈300 kJ·m-2 (Fig. 1a). The results also show that none of the DNA repair mutant strains (Table 1), such as ogg1, rad14, rad18, rad51, rad14 ogg1, rad14 rad51, rad14 ntg1 ntg2, and rad14 apn1 apn2, exhibits a sensitivity to the lethal action of UVA radiation, which significantly differs from that of the WT strain (Fig. 1a and data not shown). Therefore, NER, BER, HR, or postreplication repair pathways do not efficiently prevent the lethal action of UVA radiation in yeast. Consequently, DNA damages, such as oxidized bases, AP sites, CPDs, or DSB, are presumably not the primary or direct cause of lethality in UVA-irradiated yeast cells.
Fig. 1.
Sensitivity and mutability of DNA repair-deficient yeast strains to UVA radiation. (a) UVA sensitivity of strains deficient in BER or NER. The differences observed between the overall curves are not statistically significant (P > 0.05, linear mixed model). (b) UVA-induced CanR mutation frequencies in strains deficient in NER (rad14), NER BER (rad14 ntg1 ntg2 and rad14 apn1 apn2), and NER HR (rad14 rad51). Dose-response curves for WT, rad14, rad14 rad51, and rad14 ntg1 ntg2 do not statistically differ (P > 0.05, linear mixed model). (c) UVA-induced CanR mutation frequencies in strains deficient in BER (ogg1), NER (rad14), or both (rad14 ogg1). The ber- strain is ogg1 ntg1 ntg2 ung1 mag1. Mutation inductions in ogg1 and rad14 ogg1 significantly differ (P = 0.0286, linear mixed model). (d) UVA-induced CanR mutation frequencies in ogg1 and WT strains transformed with an empty plasmid (pYX212) or with a plasmid carrying the WT OGG1 gene (pYSB210). Spontaneous mutation frequency has been subtracted for all strains tested. Data represent the averages of at least three independent experiments. Asterisks indicate data that significantly differ after statistical analysis.
To investigate the mutagenic potency of UVA radiation, the frequency of forward CanR mutations was measured in the above yeast strains. Fig. 1b shows that the induction of CanR mutations by UVA radiation is rather low in the WT strain. Indeed, at D37 UVA radiation induces ≈10-fold less CanR mutants than UVC radiation in the WT strain (Fig. 7a, which is published as supporting information on the PNAS web site). Fig. 1b also shows that mutation induction by UVA is not enhanced in rad14, rad14 rad51, and rad14 ntg1 ntg2 mutant strains relative to the WT strain. UVA-induced mutation is slightly increased in rad14 apn1 apn2 triple-mutant cells. Fig. 1c reveals that mutation induction is much higher (≈10-fold) in the ogg1 single mutant than in the WT or in the rad14 mutant. In addition, mutation induction is similar in the ogg1 cells and in cells inactivated for all DNA glycosylases involved in BER (Ogg1p, Ntg1p, Ntg2p, Ung1p, and Mag1p), likely reflecting the lack of Ogg1p. Furthermore, UVA-induced mutation frequency in the rad14 ogg1 double mutant is significantly higher than that in the ogg1 single mutant (Fig. 1c), in agreement with a role of NER in the repair of oxidized guanines (31). The control experiment showed that the high UVA-induced mutation frequency observed in ogg1 cells is due to the absence of the Ogg1p (Fig. 1d). Therefore, our data exclude oxidized pyrimidines, AP sites, CPDs, and DSBs as the primary cause of UVA-induced mutagenesis and strongly suggest the formation of mutagenic oxidized guanine residues, which are substrates of the Ogg1p in cells exposed to UVA radiation.
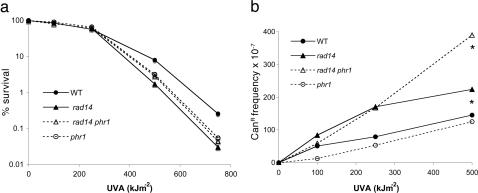
Implication of CPDs in the Genotoxic Effect of UVA Radiation. Because it has been observed that CPDs may be more frequently produced than 8-oxoG in UVA-irradiated mammalian cells (12, 14), it was worth exploring the possibility that CPDs potentially formed by UVA radiation were not subsequently reversed by the photoreactivating enzyme (Phr1p) in yeast. S. cerevisiae possesses a CPD-specific photolyase that is activated by long UVA photons. To explore this avenue, we constructed the phr1 and phr1 rad14 strains and checked that they are more sensitive to UVC radiation than the corresponding PHR1-proficient cells (data not shown). These strains were used to investigate the impact of photoreactivation on survival and mutagenesis after exposure to UVA radiation. Fig. 2a shows that phr1 and phr1 rad14 mutant strains do not exhibit increased sensitivity to UVA radiation, compared with the WT and rad14 strains. Furthermore, the four strains exhibit similar mutability at low UVA doses (Fig. 2b). At 500 kJ·m-2 of UVA, the number of mutations is twice as high in rad14 phr1 as in rad14 (P < 0.05, Wilcoxon test), suggesting that, at this dose, some CPDs are formed and photoreversed during the 10-min duration of UVA irradiation. However, even at 500 kJ·m-2 the UVA-induced mutation frequency in the phr1 rad14 mutant is 6-fold lower than that of the ogg1 rad14 mutant (Figs. 1c and 2b). Altogether, those data indicate that, at the doses used, CPDs do not contribute to the lethal effect of UVA and have a minor input in mutation induction by UVA in yeast.
Fig. 2.
Sensitivity and mutability to UVA of yeast cells deficient in CPDs photoreactivation. (a) Sensitivity of WT, phr1, rad14, and rad14 phr1 cells. (b) UVA-induced CanRmutation frequencies in WT cells and in strains deficient in NER (rad14), photoreactivating enzyme (phr1), or both (rad14 phr1). *, The mutation frequency observed at 500 kJ·m-2 in rad14 phr1 statistically differs from that in the other strains by Wilcoxon test (P < 0.05).
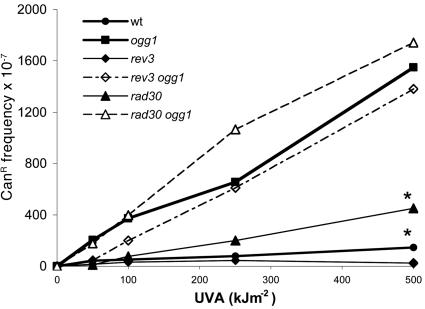
Role of Polη and Polζ in UVA Radiation-Induced Mutagenesis. To better characterize the UVA-induced mutagenesis in yeast, we examined the role of DNA polymerases involved in TLS, such as Polη (Rad30p) or Polζ (Rev3p). It is well established that Polη replicates through CPDs and 8-oxoG with high efficiency and accuracy, whereas Polζ is critically important in UVC mutagenesis (27, 28, 32, 33). Cells deficient in Polη (rad30 and rad30 ogg1) or Polζ (rev3 and rev3 ogg1) exhibit the same sensitivity to the lethal action of UVA as the WT cells (data not shown), whereas they are sensitive to UVC (28). Fig. 3 and Table 2 show that the UVA-induced CanR mutation frequency is 2- to 3-fold higher in rad30 than in WT cells. In contrast, the UVA-induced mutation frequencies are similar in the rev3 and WT strains on one hand, and mutation frequencies are similar in the ogg1 rev3 and the ogg1 mutants on the other hand (Fig. 3). Altogether, the results indicate that Polη is involved in the error-free bypass of UVA-induced mutagenic lesions, whereas Polζ plays a minor role. Again, these results point out oxidative DNA damage, such as 8-oxoG, rather than to CPDs as a major cause of UVA-induced mutagenesis in yeast.
Fig. 3.
UVA-induced CanR mutation frequencies in strains deficient in TLS polymerases, Polη(rad30), and Polζ(rev3). Mutation inductions in rad30 and in WT statistically differ (P = 0.0097, linear mixed model), whereas those in rad30 ogg1 and rev3 ogg1 do not significantly differ from that in ogg1. Asterisks indicate data that significantly differ after statistical analysis.
Table 2. Types of spontaneous and UVA-induced CanR mutations.
| Spontaneous
|
UVA, 250 kJ/m2
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation type
|
wt
|
ogg1
|
rad30
|
wt
|
ogg1
|
rad30
|
||||||
| n (%) | f × 10−7 | n (%) | f × 10−7 | n (%) | f × 10−7 | n (%) | f × 10−7 | n (%) | f × 10−7 | n (%) | f × 10−7 | |
| Single BS at | ||||||||||||
| GC | 39 (64) | 5.1 | 48 (91) | 45 | 10 (47) | 7 | 78 (75)* | 60 | 31 (97) | 708 | 16 (67) | 140 |
| AT | 12 (20) | 1.6 | 3 (6) | 3 | 6 (29) | 4.3 | 8 (8) | 6 | 1 (3) | 23 | 1 (4) | 9 |
| Transitions | ||||||||||||
| GC→AT | 20 (33) | 2.6 | 4 (8) | 4 | 3 (14) | 2.1 | 18 (17) | 14 | 2 (6) | 45 | 6 (25) | 53 |
| AT→GC | 3 (5) | 0.4 | 1 (2) | 1 | 1 (5) | 0.7 | 1 (1) | 1 | — | — | — | — |
| Transversions | ||||||||||||
| GC→TA | 11 (18) | 1.4 | 43 (81) | 40 | 7 (33) | 5 | 42 (40)** | 32 | 28 (88) | 639 | 9 (38) | 79 |
| GC→CG | 8 (13) | 1.1 | 1 (2) | 1 | — | — | 18 (17) | 14 | 1 (3)*** | 23 | 1 (4) | 9 |
| AT→CG | 7 (11) | 0.9 | — | — | 1 (5) | 0.7 | 4 (4) | 3 | 1 (3) | 23 | 1 (4) | 9 |
| AT→TA | 2 (3) | 0.3 | 2 (4) | 2 | 4 (19) | 2.9 | 3 (3) | 2 | — | — | — | — |
| 1-nt frameshift | 5 (8) | 0.7 | 2 (4) | 2 | 4 (19) | 2.9 | 14 (14) | 11 | — | — | 6 (25) | 53 |
| Others | 5 (8) | 0.7 | — | — | 1 (5) | 0.7 | 4 (4) | 4 | — | — | 1 (4) | 9 |
| Total | 61 | 8.1 | 53 | 50 | 21 | 15 | 104 | 80 | 32 | 730 | 24 | 210 |
The percentages represent the fraction of total mutational events. *, Spontaneous WT versus WT UVA (P = 0.04); **, Spontaneous WT versus WT UVA (P = 0.003); ***, WT UVA versus ogg1 UVA (P = 0.045, two-tailed Fisher's exact test). BS, base substitution, —, not found.
CanR Mutation Spectra in WT, ogg1, and rad30 Cells Exposed to UVA Radiation. If 8-oxoG is largely responsible for UVA-induced mutations in yeast, the spectrum of CanR mutation should be biased in favor of GC→TA transversions (27, 34). To challenge this hypothesis, sequence analysis of CanR mutations was performed in WT, ogg1, or rad30 cells exposed or not to 250 kJ·m-2 of UVA radiation (this UVA dose leads to 50% survival) (Table 2). In the WT strain, UVA radiation mostly induces single-base substitutions (83% of total mutations), with an excess of mutations at GC base pairs (91% of base substitutions). Remarkably, a major fraction of the UVA-induced base substitutions are GC→TA transversions. (40% of total mutations), which significantly differs from that observed in the spontaneous mutation spectrum (18%) and corresponds to a 23-fold enhanced frequency. UVA radiation also enhances the frequencies of GC→CG (13-fold) and 1-nt frame-shifts (16-fold) in the WT strain. In the ogg1 mutant strain, GC→TA is by far the major class of mutations and represents 81% and 88% of all spontaneous and UVA-induced CanR mutations, respectively. In the rad30 strain, the frequency of UVA-induced GC→TA is 2.5-fold higher than in the WT strain, and it is 16-fold higher than the spontaneous mutation. These last results are consistent with the involvement of Polη in the error-free bypass of UVA-induced mutagenic DNA damage. Table 2 also shows that the frequency of GC→AT transitions, possibly because of CPDs, is moderately increased in rad30 strain in comparison to WT after UVA irradiation. This last observation confirms that CPDs are not an important source of UVA-induced mutations in yeast. Figs. 8 and 9, which are published as supporting information on the PNAS web site, show that UVA-induced mutational events are rather dispersed along the CAN1 gene, and the GC→TA occurring spontaneously or after UVA irradiation are located at the same sites in WT and ogg1. Altogether, the high frequency of GC→TA events in WT and rad30 strains and the almost exclusive contribution of this event in the ogg1 strain strongly suggest 8-oxoG as the predominant mutagenic lesion induced by UVA in yeast.
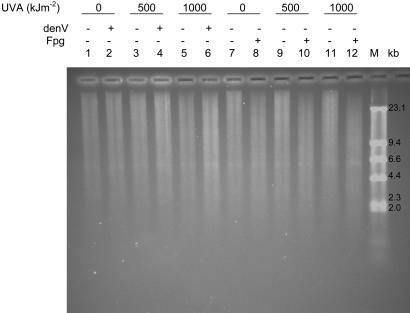
8-oxoG Formation Is the Major DNA Damage Induced by UVA Radiation. We explored the formation of CPDs and 8-oxoG in cellular DNA of UVA-irradiated rad14 ogg1 mutant yeast, using alkaline agarose gel in combination with specific DNA repair enzymes. The T4 denV protein cleaves DNA at CPDs and the E. coli Fpg protein incises DNA at oxidative DNA lesions, mainly 8-oxoG. Fig. 4 shows that the genomic DNA from unirradiated cells migrates as a smear (lanes 1 and 7). The T4 denV protein did not create detectable additional breaks above those observed by mock and UVA treatment alone (lanes 2, 4, and 6). Similarly, CPDs could not be detected in UVA-irradiated rad14 phr1 mutant cells (data not shown). In contrast, Fpg-sensitive sites increase with increasing UVA doses (lanes 8, 10, and 12). These results are in excellent agreement with those presented above and demonstrate that CPDs are very poorly produced by UVA in yeast, whereas 8-oxoG is fairly induced.
Fig. 4.
Detection of CPDs and Fpg-sensitive sites in UVA-irradiated yeast DNA. The rad14 ogg1 yeast cells were exposed or not to 500 or 1,000 kJ·m-2 UVA radiation. DNA was extracted and incubated in the absence or presence of T4 denV (1,000 units) or Fpg protein (250 ng) and analyzed by alkaline agarose gel electrophoresis. Quantification performed as described in ref. 12 indicates that the number of Fpg-sensitive sites observed per 106 bp of DNA from unirradiated cells or from cells receiving 500 or 1,000 kJ·m-2 UVA is 15, 26, and 40, respectively.
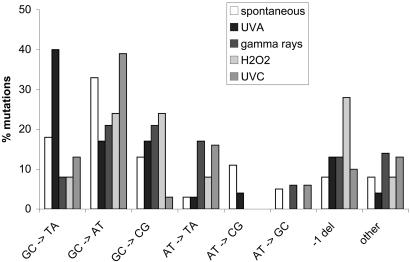
UVA-Induced Mutagenesis Is Specific and Differs from That of UVC, H2O2, and γ-Rays. We analyzed the sensitivity and mutability of WT and ogg1 cells to UVC, H2O2, and γ-rays in comparison to UVA radiation. In the WT strain, at equal survival, UVC, H2O2, and γ-rays are potent mutagens, whereas UVA is poorly mutagenic (Fig. 7a). In the ogg1 mutant, the induction of CanR mutations by UVC, H2O2, and γ-rays does not change comparatively to that observed in the WT strain (Fig. 7b). In contrast, at equal survival, UVA radiation generates many more CanR mutations in the ogg1 strain than in the WT (Fig. 7b). It should be noted that ogg1 strains are not unusually sensitive to the lethal action of UVA, UVC, H2O2, or γ-rays, compared with the WT (Fig. 1a and data not shown). Therefore, the hypersensitivity of ogg1 mutants to the mutagenic action of UVA radiation strongly suggests that the pattern of DNA lesions induced by UVA significantly differs from those induced by UVC, H2O2, and γ-rays. As pointed out in Fig. 5, the mutational specificity of UVA radiation in WT yeast is rather unique and is characterized by a majority of base substitutions at GC pairs and primarily GC→TA transversions. Interestingly, UVA mutation spectrum differs from that of the oxidizing agent H2O2 or that of ionizing radiation, both characterized by a low level of GC→TA (Fig. 5). Moreover, the mutational specificity of UVA is not of a UVC type, which is characterized by a majority of GC→AT transitions because of bipyrimidine photoproducts. These data strongly suggest that UVA radiation generates reactive species whose chemical nature and/or relative amounts significantly differ from those created by other oxidants, such as H2O2 or γ-radiation.
Fig. 5.
CanR mutation spectrum in WT yeast. The types of spontaneous mutations (empty bars) and mutations induced by UVA radiation (250 kJ·m-2), γ-radiation (600 Gy), H2O2 (6 mM), or UVC radiation (100 J·m-2) are shown. Values are expressed as percent of total mutations. Number of mutants analyzed is as follows: 61 for spontaneous mutations, 104 for UVA radiation, 37 for γ-radiation, 25 for H2O2, and 31 for UVC radiation.
Discussion
In the present study, we investigated the impact of UVA radiation on survival and mutagenesis in the yeast S. cerevisiae. In general, yeast can be used as a relevant model for all eukaryotes in the fields of DNA repair and mutagenesis. We first analyzed the role of the major DNA repair pathways on the lethal action of UVA radiation. The results show that the inactivation of DNA repair pathways, such as BER, NER, HR, postreplication repair, or TLS does not sensitize yeast cells to the lethal action of UVA radiation. However, UVA radiation has a significant effect on cell survival at the doses used in the present study. At 500 kJ·m-2, the highest dose used, the fraction of surviving cells was ≈10% for the WT and all of the mutants tested, including mutants deficient in more than one repair pathway, i.e., NER-BER- or NER-HR-. Importantly, these DNA repair mutants are all extremely sensitive to the killing effect of classical DNA-damaging agents, and consequently they are able to reveal the formation of DNA lesions that impair DNA replication, such as oxidized bases, AP sites, CPDs, or DSBs. Our observations could thus suggest that UVA radiation does not generate detectable amounts of DNA damage, which is compatible with the low capacity of UVA photons to directly damage DNA. Nonetheless, the biological effects of UVA have mostly been ascribed to endogenous photosensitizers yielding singlet oxygen and type-1 photo-sensitization reactions, which, in turn, could damage DNA (5). Indeed, DNA damage, such as 8-oxoG and CPDs, have been observed in mammalian cells exposed to UVA radiation (9). Assuming that UVA induces DNA damage in yeast, it could mostly produce lesions that are well tolerated by the replication machinery, such as 8-oxoG or uracil derivatives, as suggested by the lack of sensitivity of yeast DNA repair mutants. Besides damaging DNA, UVA radiation also causes damage to proteins and lipids in various cellular compartments and modifies cellular redox state (5, 35, 36). Indeed, non-DNA damage is likely the main cause of cell death after UVA irradiation and hides the effect of cytotoxic DNA lesions. To the best of our knowledge, the only yeast mutants unusually sensitive to the lethal effect of UVA are not affected in DNA repair but in the phosphotyrosyl phosphatase activator protein (Ptpa1p) or in the threonine/serine phosphatase (Sit4p) (37, 38). These authors suggest that UVA radiation triggers Swe1p-induced hyperphosphorylation of Cdc28p to arrest cells in the G1 and G2/M phases. In the absence of Ptpa1p or Sit1p, cell cycle arrest would persist and lead to cell death. The UVA-induced cellular lesion at the origin of the signalization pathway leading to cell cycle arrest remains unknown. A role for DNA damage cannot be excluded.
We explored the mutagenic potency and specificity of UVA radiation in WT and mutant yeast strains affected in DNA repair and translesion synthesis. All of our results point out oxidized purines, most likely 8-oxoG, as the major UVA-induced mutagenic lesion in yeast, whereas CPDs and other lesions play a minor role. Indeed, only yeast cells deficient in the Ogg1p, a DNA glycosylase that excises 8-oxoG and 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) (39), are unambiguously hypermutable upon exposure to UVA radiation. The mutation spectrum in WT cells exposed to UVA radiation is predominated by GC→TA transversions, those events being almost exclusive in ogg1 mutant cells. Oxidation or reduction products of pyrimidines that are substrates of Ntg1 and Ntg2 glycosylases and NER (40) do not significantly contribute to the genotoxicity of UVA in yeast, indicating a minor role of hydroxyl radicals in the induction of DNA lesions by UVA radiation (41). Furthermore, the fact that NER- (rad14) and NER-HR- (rad14 rad51) strains are not unusually sensitive to or mutable by UVA radiation compared with the WT strain strongly argues against a major role of CPDs and DSBs in yeast. In S. cerevisiae exists the possibility that CPDs formed by UVA radiation are subsequently photoreversed by the photoreactivating enzyme (Phr1p). However, our results demonstrate that even in a rad14 phr1 double mutant, any contribution of CPDs to UVA-induced mutagenesis is of minor importance compared with that of 8-oxoG. In line with this finding, CPDs were not detected in UVA-irradiated yeast cells, whereas the formation of 8-oxoG was supported by the detection of Fpg-sensitive sites in UVA-irradiated DNA. Finally, the spectrum of CanR mutations induced by UVA radiation is clearly different from that induced by UVC radiation. UVA-induced CanR mutations also differ from those induced by H2O2 and γ-radiation. These data establish that the nature and/or the relative amounts of UVA-induced reactive species significantly differ from that of other oxidants, such as H2O2 and γ-rays. Indeed, UVA radiation mostly induces base substitutions at GC pairs with an excess of GC→TA and GC→CG events. This pattern is similar to that obtained at the gpt locus after E. coli transfection of plasmid DNA exposed in vitro to treatments that primarily generate singlet oxygen (42). Therefore, the data strongly suggest that, in yeast, UVA radiation causes the formation of DNA lesions and, in particular, 8-oxoG by means of the action of singlet oxygen rather than that of hydroxyl radicals.
In mammalian cells, we previously observed that CPDs are more frequently produced by UVA radiation than 8-oxoG, other DNA damage being poorly represented (10, 12). Moreover, the currently available investigations on UVA mutagenesis generated very conflicting results, some reporting a majority of mutations at 8-oxoG (15) and others reporting a majority of mutations at CPDs (13, 16-18, 43). One can argue that those discrepancies arose from different experimental conditions for UVA irradiation, damage detection, and mutagenesis. These assertions surely contribute to a certain extent to the differences observed. However, the differences found between yeast and mouse fibroblasts on one side and Chinese hamster ovary cells, human embryonic kidney cells, primary cultured human fibroblasts, and keratinocytes (14) on the other side suggest that, depending on the cell type and content of endogenous photosensitizers, a finely tuned interplay between UVA radiation and photosensitizers can determine the subsequent cytotoxicity and mutagenesis. Another important factor is the repair timing and efficiency of 8-oxoG versus CPDs, which may vary in the different cell types. An important contribution will be to investigate UVA mutagenesis in mammalian cells whose OGG1 activity has been depleted (44).
In conclusion, our data in yeast demonstrate that UVA radiation can be strongly mutagenic because of the formation of oxidative DNA damage and thus may play a pivotal role in the malignant transformation of human skin. The data emphasize the need to reconsider the mode of protection against sunlight and to better preserve the human population from UVA exposure. An effective protection would require the use of UVA blocking agents associated with an antioxidant strategy.
Supplementary Material
Acknowledgments
We thank Samira Makzami for technical assistance. This work was supported by the Centre National de la Recherche Scientifique, the Institut Curie, the Commissariat à l'Energie Atomique, and the Association pour la Recherche sur le Cancer.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AP, abasic; BER, base excision repair; CanR, canavanine-resistant; CPD, cyclobutane pyrimidine dimer; DSB, double-stranded break; HR, homologous recombination; NER, nucleotide excision repair; TLS, translesion DNA synthesis; 8-oxoG, 7,8-dihydro-8-oxoguanine; YPD, yeast extract/peptone/dextrose; YNBD, yeast nitrogen base and dextrose.
References
- 1.van Weelden, H., van der Putte, S. C., Toonstra, J. & van der Leun, J. C. (1990) Arch. Dermatol. Res. 282, 289-294. [DOI] [PubMed] [Google Scholar]
- 2.Urbach, F. (1993) Recent Results Cancer Res. 128, 243-262. [DOI] [PubMed] [Google Scholar]
- 3.Gasparro, F. P. (2000) Environ. Health Perspect. 108, Suppl. 1, 71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, H. S., deGruijl, F. R., Forbes, P. D., Cleaver, J. E., Ananthaswamy, H. N., deFabo, E. C., Ullrich, S. E. & Tyrrell, R. M. (1997) J. Photochem. Photobiol. B 40, 29-47. [DOI] [PubMed] [Google Scholar]
- 5.Tyrrell, R. M. & Keyse, S. M. (1990) J. Photochem. Photobiol. B 4, 349-361. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, X., Rosenstein, B. S., Wang, Y., Lebwohl, M., Mitchell, D. M. & Wei, H. (1997) Photochem. Photobiol. 65, 119-124. [DOI] [PubMed] [Google Scholar]
- 7.Kvam, E. & Tyrrell, R. M. (1997) Carcinogenesis 18, 2379-2384. [DOI] [PubMed] [Google Scholar]
- 8.Kielbassa, C., Roza, L. & Epe, B. (1997) Carcinogenesis 18, 811-816. [DOI] [PubMed] [Google Scholar]
- 9.Cadet, J., Sage, E. & Douki, T. (2005) Mutat. Res. 571, 3-17. [DOI] [PubMed] [Google Scholar]
- 10.Douki, T., Perdiz, D., Grof, P., Kuluncsics, Z., Moustacchi, E., Cadet, J. & Sage, E. (1999) Photochem. Photobiol. 70, 184-190. [PubMed] [Google Scholar]
- 11.Perdiz, D., Grof, P., Mezzina, M., Nikaido, O., Moustacchi, E. & Sage, E. (2000) J. Biol. Chem. 275, 26732-26742. [DOI] [PubMed] [Google Scholar]
- 12.Douki, T., Reynaud-Angelin, A., Cadet, J. & Sage, E. (2003) Biochemistry 42, 9221-9226. [DOI] [PubMed] [Google Scholar]
- 13.Rochette, P. J., Therrien, J. P., Drouin, R., Perdiz, D., Bastien, N., Drobetsky, E. A. & Sage, E. (2003) Nucleic Acids Res. 31, 2786-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courdavault, S., Baudouin, C., Charveron, M., Favier, A., Cadet, J. & Douki, T. (2004) Mutat. Res. 556, 135-142. [DOI] [PubMed] [Google Scholar]
- 15.Besaratinia, A., Synold, T. W., Xi, B. & Pfeifer, G. P. (2004) Biochemistry 43, 8169-8177. [DOI] [PubMed] [Google Scholar]
- 16.Drobetsky, E. A., Turcotte, J. & Chateauneuf, A. (1995) Proc. Natl. Acad. Sci. USA 92, 2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert, C., Muel, B., Benoit, A., Dubertret, L., Sarasin, A. & Stary, A. (1996) J. Invest. Dermatol. 106, 721-728. [DOI] [PubMed] [Google Scholar]
- 18.Sage, E., Lamolet, B., Brulay, E., Moustacchi, E., Chteauneuf, A. & Drobetsky, E. A. (1996) Proc. Natl. Acad. Sci. USA 93, 176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agar, N. S., Halliday, G. M., Barnetson, R. S., Ananthaswamy, H. N., Wheeler, M. & Jones, A. M. (2004) Proc. Natl. Acad. Sci. USA 101, 4954-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard, P. M. & Boiteux, S. (1997) Biochimie 79, 559-566. [DOI] [PubMed] [Google Scholar]
- 21.Boiteux, S. & Guillet, M. (2004) DNA Repair 3, 1-12. [DOI] [PubMed] [Google Scholar]
- 22.Resnick, M. A. & Cox, B. S. (2000) Mutat. Res. 451, 1-11. [DOI] [PubMed] [Google Scholar]
- 23.Friedberg, E., Walker, G. & Siede, W. (1995) (Am. Soc. Microbiol., Washington, DC).
- 24.Wach, A., Brachat, A., Alberti-Segui, C., Rebischung, C. & Philippsen, P. (1997) Yeast 13, 1065-1075. [DOI] [PubMed] [Google Scholar]
- 25.Longtine, M. S., McKenzie, A., III, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P. & Pringle, J. R. (1998) Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- 26.Lea, D. E. & Coulson, D. A. (1949) J. Genet. 49, 264-285. [DOI] [PubMed] [Google Scholar]
- 27.de Padula, M., Slezak, G., Auffret van Der Kemp, P. & Boiteux, S. (2004) Nucleic Acids Res. 32, 5003-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozmin, S. G., Pavlov, Y. I., Kunkel, T. A. & Sage, E. (2003) Nucleic Acids Res. 31, 4541-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravanat, J. L., Douki, T., Duez, P., Gremaud, E., Herbert, K., Hofer, T., Lasserre, L., Saint-Pierre, C., Favier, A. & Cadet, J. (2002) Carcinogenesis 23, 1911-1918. [DOI] [PubMed] [Google Scholar]
- 30.Cnaan, A., Laird, N. M. & Slasor, P. (1997) Stat. Med. 16, 2349-2380. [DOI] [PubMed] [Google Scholar]
- 31.Scott, A. D., Neishabury, M., Jones, D. H., Reed, S. H., Boiteux, S. & Waters, R. (1999) Yeast 15, 205-218. [DOI] [PubMed] [Google Scholar]
- 32.Morrison, A., Christensen, R. B., Alley, J., Beck, A. K., Bernstine, E. G., Lemontt, J. F. & Lawrence, C. W. (1989) J. Bacteriol. 171, 5659-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haracska, L., Yu, S. L., Johnson, R. E., Prakash, L. & Prakash, S. (2000) Nat. Genet. 25, 458-461. [DOI] [PubMed] [Google Scholar]
- 34.Ni, T. T., Marsischky, G. T. & Kolodner, R. D. (1999) Mol. Cell 4, 439-444. [DOI] [PubMed] [Google Scholar]
- 35.Morliere, P., Moysan, A., Santus, R., Huppe, G., Maziere, J. C. & Dubertret, L. (1991) Biochim. Biophys. Acta 1084, 261-268. [DOI] [PubMed] [Google Scholar]
- 36.Budai, M., Reynaud-Angelin, A., Szabo, Z., Toth, S., Ronto, G., Sage, E. & Grof, P. (2004) J. Photochem. Photobiol. B 77, 27-38. [DOI] [PubMed] [Google Scholar]
- 37.Ramotar, D., Belanger, E., Brodeur, I., Masson, J. Y. & Drobetsky, E. A. (1998) J. Biol. Chem. 273, 21489-21496. [DOI] [PubMed] [Google Scholar]
- 38.Douville, J., David, J., Fortier, P. K. & Ramotar, D. (2004) Curr. Genet. 46, 72-81. [DOI] [PubMed] [Google Scholar]
- 39.Boiteux, S., Gellon, L. & Guibourt, N. (2002) Free Radical Biol. Med. 32, 1244-1253. [DOI] [PubMed] [Google Scholar]
- 40.Gellon, L., Barbey, R., Auffret van der Kemp, P., Thomas, D. & Boiteux, S. (2001) Mol. Genet. Genomics 265, 1087-1096. [DOI] [PubMed] [Google Scholar]
- 41.Pouget, J. P., Douki, T., Richard, M. J. & Cadet, J. (2000) Chem. Res. Toxicol. 13, 541-549. [DOI] [PubMed] [Google Scholar]
- 42.Schulz, I., Mahler, H. C., Boiteux, S. & Epe, B. (2000) Mutat. Res. 461, 145-156. [DOI] [PubMed] [Google Scholar]
- 43.Ikehata, H., Nakamura, S., Asamura, T. & Ono, T. (2004) Mutat. Res. 556, 11-24. [DOI] [PubMed] [Google Scholar]
- 44.Klungland, A., Rosewell, I., Hollenbach, S., Larsen, E., Daly, G., Epe, B., Seeberg, E., Lindahl, T. & Barnes, D. E. (1999) Proc. Natl. Acad. Sci. USA 96, 13300-13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.