Abstract
background
The magnitude and scope of Clostridium difficile infection in the United States continue to evolve.
methods
In 2011, we performed active population- and laboratory-based surveillance across 10 geographic areas in the United States to identify cases of C. difficile infection (stool specimens positive for C. difficile on either toxin or molecular assay in residents ≥1 year of age). Cases were classified as community-associated or health care–associated. In a sample of cases of C. difficile infection, specimens were cultured and isolates underwent molecular typing. We used regression models to calculate estimates of national incidence and total number of infections, first recurrences, and deaths within 30 days after the diagnosis of C. difficile infection.
results
A total of 15,461 cases of C. difficile infection were identified in the 10 geographic areas; 65.8% were health care–associated, but only 24.2% had onset during hospitalization. After adjustment for predictors of disease incidence, the estimated number of incident C. difficile infections in the United States was 453,000 (95% confidence interval [CI], 397,100 to 508,500). The incidence was estimated to be higher among females (rate ratio, 1.26; 95% CI, 1.25 to 1.27), whites (rate ratio, 1.72; 95% CI, 1.56 to 2.0), and persons 65 years of age or older (rate ratio, 8.65; 95% CI, 8.16 to 9.31). The estimated number of first recurrences of C. difficile infection was 83,000 (95% CI, 57,000 to 108,900), and the estimated number of deaths was 29,300 (95% CI, 16,500 to 42,100). The North American pulsed-field gel electrophoresis type 1 (NAP1) strain was more prevalent among health care–associated infections than among community-associated infections (30.7% vs. 18.8%, P<0.001)
conclusions
C. difficile was responsible for almost half a million infections and was associated with approximately 29,000 deaths in 2011. (Funded by the Centers for Disease Control and Prevention.)
Changes in the epidemiology of CLOStridium difficile infections have occurred since the emergence of the North American pulsed-field gel electrophoresis type 1 (NAP1) strain, which has been responsible for geographically dispersed hospital-associated outbreaks.1–3 In the United States, hospitalizations for C. difficile infection among nonpregnant adults doubled from 2000 through 2010 and were projected to continue to increase in 2011 and 2012, especially as laboratories transition to more sensitive C. difficile assays, such as the nucleic acid amplification test (NAAT).4–6 On the basis of data from U.S. death certificates, C. difficile infection is the leading cause of gastroenteritis-associated death and was estimated to cause 14,000 deaths in 2007.7 C. difficile has become the most common cause of health care–associated infections in U.S. hospitals, and the excess health care costs related to C. difficile infection are estimated to be as much as $4.8 billion for acute care facilities alone.8–10 In addition, C. difficile infection has been increasingly reported outside of acute care facilities, including in community and nursing homes settings, where infection may be diagnosed and treated without hospitalization.11–13 As the epidemiology of C. difficile changes, both in health care and community settings, it is important to understand the magnitude and scope of this infection in the United States to help guide priorities for prevention.
In 2009, the Centers for Disease Control and Prevention (CDC) started active population- and laboratory-based surveillance for C. difficile infection at 7 U.S. sites. This surveillance was expanded to 10 sites in 2011 to provide better national estimates of disease burden, incidence, recurrence, and mortality by capturing data across the spectrum of health care delivery and community settings.
METHODS
SURVEILLANCE POPULATION AND DEFINITION
C. difficile surveillance is a component of the CDC’s Emerging Infections Program (EIP). In 2011, C. difficile surveillance was conducted at 10 EIP sites across 34 counties (total population, approximately 11.2 million) for the entire calendar year. Surveillance catchment areas included California (1 urban county; population, 812,826), Colorado (5 urban counties; population, 2,488,410), Connecticut (1 urban county; population, 861,113), Georgia (8 urban counties; population, 3,753,452), Maryland (3 rural and 8 urban counties; population, 835,893), Minnesota (2 rural and 2 urban counties; population, 248,079), New Mexico (1 urban county; population, 670,968), New York (1 urban county; population, 745,625), Oregon (1 rural county; population, 66,299), and Tennessee (1 urban county; population, 635,475).
The surveillance methods have been described previously.14,15 Briefly, surveillance staff at each EIP site identified all positive C. difficile test results from 88 inpatient and 33 outpatient laboratories serving residents in surveillance areas in 2011. A case of C. difficile infection was defined as a positive result on a C. difficile toxin or molecular assay of a stool specimen obtained from a surveillance-area resident at least 1 year of age who had not had a positive assay in the previous 8 weeks (i.e., incident infection). This surveillance was approved by the institutional review boards at the CDC and at the participating EIP sites.
data collection
We performed an initial medical-record review to collect data on demographic characteristics, the location of stool collections, and health care exposures on all cases of C. difficile infection in 8 of the 10 EIP sites. In 2 EIP sites with the largest surveillance populations (Georgia and Colorado), we performed an initial medical-record review on a random sample of cases, as described previously.15
On the basis of the initial medical review, a case was classified as community-associated if the C. difficile–positive specimen was collected on an outpatient basis or within 3 days after hospital admission and the patient had no documented overnight stay in a health care facility during the previous 12 weeks. All other cases were classified as health care–associated and further categorized into three mutually exclusive groups: community onset associated with a health care facility, hospital onset, or nursing home onset (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). All cases that were classified as either community-associated or community-onset health care–associated underwent full medical-record review to collect information on coexisting medical conditions, medication exposures, first laboratory-confirmed recurrences (i.e., positive specimen within 2 to 8 weeks after the last positive test), and death within 30 days after diagnosis of C. difficile infection. In addition, we reviewed a sample consisting of 10% of cases with an onset in a nursing home or hospital.
A convenience sample of clinical laboratories across the EIP sites (37 laboratories) submitted all C. difficile–positive stool specimens from cases with full medical-record review for culture.16 Recovered isolates underwent pulsed-field gel electrophoresis (PFGE). PFGE patterns were analyzed with the use of BioNumerics software, version 5.10 (Applied Maths) and grouped into pulsed-field types with the use of Dice coefficient analysis and UPGMA (unweighted pair group method with arithmetic mean) clustering. An 80% similarity threshold was used to assign North American PFGE (NAP) types.17 Isolates also underwent polymerase-chain-reaction (PCR) assay to detect the presence of tcdA, tcdB, and binary toxin (cdtA and ctdB) genes and a subset of the most common NAP types underwent PCR ribotyping.18
Between November 2011 and January 2012, all laboratories serving the surveillance population were surveyed to assess the type of C. difficile diagnostic tests that were used during 2011.19 Laboratory surveys were used to estimate the proportion of cases in the surveillance areas that were identified by means of NAAT.
statistical analysis
Data were analyzed with the use of SAS software, version 9.3 (SAS Institute). In cases of C. difficile infection in which the patient’s race was unknown (18.7%), including sampled cases from Georgia and Colorado, we imputed race on the basis of the distribution of known races according to age, sex, and surveillance site.20 After race imputation was performed, a domain (subpopulation) analysis was used to estimate the number of cases according to epidemiologic class and race in the two EIP sites where sampling was performed (Georgia and Colorado).21
To generate an estimate of the national burden of C. difficile infection, we built two generalized linear mixed models with negative binomial distribution, one for health care–associated cases and another for community-associated cases, using predictors that had been shown to be associated with infection incidence in each epidemiologic category.15 We estimated the national number of health care–associated infections using model coefficients that accounted for the age of the population, the volume of inpatient days, and the proportion of cases identified by means of NAAT across EIP sites, since the rate of NAAT use in the United States is unknown. We estimated the national number of community-associated cases in a similar way, accounting for age, sex, and race of the U.S. population, as well as NAAT use across the EIP sites. We constructed 95% confidence intervals for the national estimates according to each epidemiologic category using imputation error, sampling error for Georgia and Colorado, and modeling error.20,21 We then calculated the total national burden of C. difficile infection by adding estimated numbers of community-associated and health care–associated cases and 95% confidence intervals.
We estimated the numbers of recurrences and deaths within 30 days and corresponding 95% confidence intervals by performing domain analysis21 to account for sampling design across EIP sites and using site-specific and national sampling weights for the national projections. We calculated the population-based incidence of C. difficile infection (site-specific and national) using 2011 U.S. Census data.22 In this calculation, we excluded infants under the age of 1 year from the denominator, since they were not included in the numerator. We also performed a sensitivity analysis to estimate the national burden of C. difficile infection according to different levels of NAAT use.
RESULTS
incidence and burden of C. DIFFICILE infection
From January 1, 2011, to December 31, 2011, we identified 15,461 cases of C. difficile infection in 14,453 patients across the 10 EIP sites. Of these cases, 65.8% were health care–associated, and 24.2% were hospital-onset. The crude incidence per 100,000 population ranged from 30 to 120 cases of community-associated infection and from 50 to 160 cases of health care–associated infection across the EIP sites. The incidence of health care–associated infection was higher than the incidence of community-associated infection for all sites except Minnesota, where the surveillance population was primarily rural (Table 1).
Table 1. Incidence of Clostridium difficile Infection (CDI), According to Geographic Location and Epidemiologic Category, 2011.
| Site | Counties under Surveillance | Population ≥1 Yr of Age | Community-Associated CDI | Health Care–Associated CDI | ||
|---|---|---|---|---|---|---|
| no. | Total No. of Cases | Incidence per 100,000 Persons | Total No. of Cases | Incidence per 100,000 Persons | ||
| All sites | 10,971,319 | 5284 | 48.2 | 10,177 | 92.8 | |
| California | San Francisco | 804,110 | 297 | 37.0 | 733 | 91.1 |
| Colorado† | Adams, Arapahoe, Denver, Douglas, Jefferson | 2,454,142 | 1229 | 50.1 | 2,200 | 89.7 |
| Connecticut | New Haven | 851,962 | 393 | 46.1 | 1,355 | 159.1 |
| Georgia† | Clayton, Cobb, Douglas, DeKalb, Fulton, Gwinnett, Newton, Rockdale | 3,699,307 | 1395 | 37.7 | 2,381 | 64.7 |
| Maryland | Caroline, Cecil, Dorchester, Frederick, Kent, Somerset, Talbot, Queen Anne’s, Washington, Wicomico, Worcester | 826,430 | 485 | 58.7 | 1,056 | 127.7 |
| Minnesota | Stearns, Benton, Morrison, Todd | 244,884 | 303 | 123.7 | 177 | 72.3 |
| New Mexico | Bernalillo | 661,779 | 354 | 53.4 | 727 | 109.9 |
| New York | Monroe | 737,270 | 634 | 86.0 | 1,145 | 155.3 |
| Oregon | Klamath | 65,545 | 27 | 41.2 | 31 | 47.3 |
| Tennessee | Davidson | 625,890 | 167 | 26.7 | 372 | 59.4 |
The 2011 population is based on estimates from the U.S. Census Bureau.22 The epidemiologic category was statistically imputed for cases with unknown epidemiologic data as follows: 3 cases in California, 39 cases in Maryland, and 43 cases in New Mexico.
The weighted frequency of cases was based on 33% random sampling.
The pooled mean crude incidence of community-associated infection was 48.2 per 100,000 population. After accounting for age, sex, and race of the U.S. population and NAAT use across EIP sites, the national estimated incidence of community-associated C. difficile infection was 51.9 (95% confidence interval [CI], 43.2 to 60.5) per 100,000 population, for a national burden estimate of 159,700 cases (95% CI, 132,900 to 186,000). For health care–associated infection, the pooled mean crude incidence was 92.8 cases per 100,000 population. After accounting for the age of the U.S. population, the volume of inpatient days, and a presumed NAAT use of 52% on the basis of the EIP sites, the national estimated incidence of health care–associated C. difficile infection was 95.3 (95% CI, 85.9 to 104.8) per 100,000 population, for a national burden estimate of 293,300 cases (95% CI, 264,200 to 322,500). Overall, we estimated that 453,000 cases of C. difficile infection (95% CI, 397,100 to 508,500) occurred in 2011 (Table 2). Incidence estimates were higher among females than among males (rate ratio, 1.26; 95% CI, 1.25 to 1.27), among whites than among nonwhites (rate ratio, 1.72; 95% CI, 1.56 to 2.00), and among persons 65 years of age or older than among those under the age of 65 years (rate ratio, 8.65; 95% CI, 8.16 to 9.31).
Table 2. Adjusted U.S. National Estimates of Burden and Incidence of CDI, 2011.
| Demographic Characteristic | Community-Associated CDI* | Health Care–Associated CDI† | All CDI | |||
|---|---|---|---|---|---|---|
| Estimated No. of Cases | Incidence per 100,000 Persons | Estimated No. of Cases | Incidence per 100,000 Persons | Estimated No. of Cases | Incidence per 100,000 Persons | |
| All cases | 159,700 (132,900–186,000) | 51.9 (43.2–60.5) | 293,300 (264,200–322,500) | 95.3 (85.9–104.8) | 453,000 (397,100–508,500) | 147.2 (129.1–165.3) |
| Sex | ||||||
| Male | 64,300 (52,800–75,300) | 42.5 (34.8–49.8) | 132,700 (118,700–146,700) | 87.7 (78.5–97.0) | 197,000 (171,500–222,000) | 130.2 (113.3–146.8) |
| Female | 95,400 (80,100–110,700) | 61.0 (51.2–70.8) | 160,600 (145,500–175,800) | 102.7 (93.1–112.5) | 256,000 (225,600–286,500) | 163.8 (144.3–183.3) |
| Age group | ||||||
| 1–17 yr | 12,500 (10,000–15,000) | 17.9 (14.1–21.4) | 4400 (3200–5800) | 6.3 (4.6–8.3) | 16,900 (13,200–20,800) | 24.2 (18.7–29.7) |
| 18–44 yr | 35,600 (26,000–39,200) | 28.7 (22.9–34.5) | 20,800 (16,700–24,800) | 18.3 (14.7–21.9) | 53,400 (42,700–64,000) | 47.0 (37.6–56.4) |
| 45–64 yr | 54,100 (45,600–62,600) | 65.4 (55.1–75.6) | 68,800 (61,000–76,600) | 83.1 (73.7–92.5) | 122,900 (106,600–139,200) | 148.5 (128.8–168.1) |
| ≥65 yr | 60,500 (51,300–69,200) | 146.2 (124.0–167.2) | 193,300 (183,300–215,300) | 481.5 (442.8–520.1) | 259,800 (234,600–284,500) | 627.7 (566.8–687.3) |
| Race‡ | ||||||
| White | 138,100 (118,500–157,700) | 57.4 (49.2–65.5) | 259,900 (230,100–273,800) | 104.7 (95.6–113.8) | 390,000 (348,600–431,500) | 162.1 (144.8–179.3) |
| Nonwhite | 21,600 (14,400–28,300) | 32.2 (21.5–42.2) | 41,400 (34,100–48,700) | 61.8 (50.9–72.7) | 63,000 (48,500–77,000) | 94.0 (72.4–114.9) |
Data for community-associated Clostridium difficile infection (CDI) were adjusted for age, sex, race, and a rate of use of nucleic acid amplification test (NAAT) of 52%. Ranges in parentheses are 95% confidence intervals.
Data for health care–associated CDI were adjusted for age, inpatient days, and a rate of use of NAAT of 52%.
Race was imputed for 18.7% of the observed cases of C. difficile infection.
Of the 293,300 health care–associated cases, we estimated that 107,600 (95% CI, 97,200 to 118,000) had a hospital onset, 104,400 (95% CI, 94,100 to 115,800) had a nursing home onset, and 81,300 (95% CI, 72,900 to 89,000) had a community onset associated with a health care facility (Fig. 1).
Figure 1. Estimated U.S. Burden of Clostridium difficile Infection (CDI), According to the Location of Stool Collection and Inpatient Health Care Exposure, 2011.
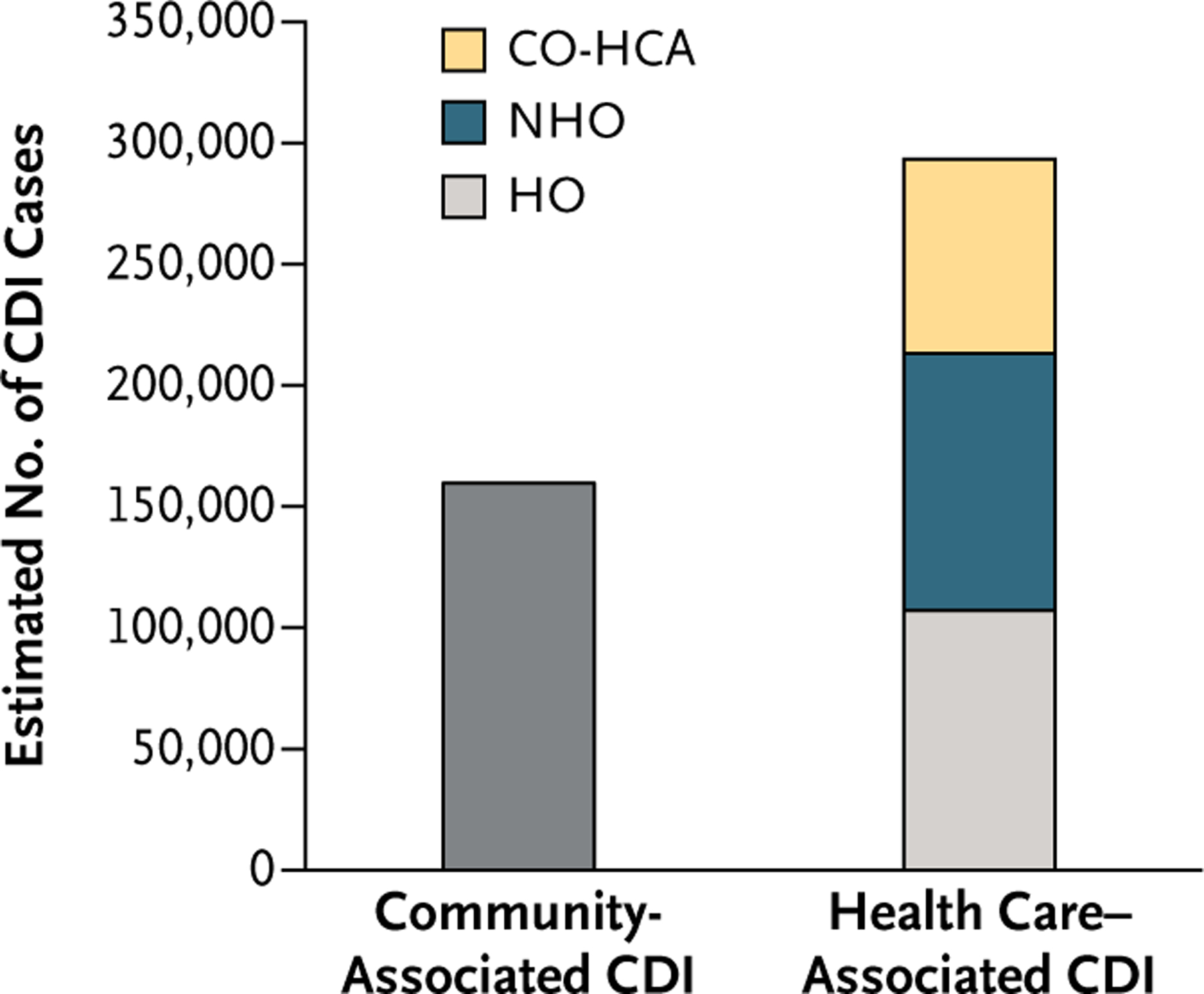
Of the estimated cases of community-associated CDI, 82% were estimated to be associated with outpatient health care exposure.11 CO-HCA denotes community-onset health care–associated infection, HO hospital onset, and NHO nursing home onset.
As determined on sensitivity analysis, the national estimates of health care–associated, community-associated, and overall infection burden could change substantially, depending on NAAT use, ranging from a total of 325,300 cases (95% CI, 286,300 to 364,000) if no U.S. laboratories were using NAAT to 622,600 cases (95% CI, 543,400 to 701,100) if all U.S. laboratories adopted NAAT (Fig. S1 in the Supplementary Appendix).
C. difficile recurrence and mortality
Among the cases of community-associated infection, the estimated rate was 13.5% for first recurrence and 1.3% for death within 30 days after diagnosis of C. difficile infection, for national estimates of 21,600 first recurrences (95% CI, 16,900 to 26,300) and 2000 deaths (95% CI, 1200 to 2800). Recurrence and death were more commonly observed among the health care–associated infections than among community-associated infections. Of the patients with health care–associated infection, the rate of first recurrence was estimated at 20.9%, and the rate of death within 30 days was 9.3%, resulting in an estimated 61,400 recurrences (95% CI, 40,200 to 82,600) and 27,300 deaths (95% CI, 15,300 to 39,300) nationally (Table 3).
Table 3. Adjusted U.S. National Estimates of Recurrences and Deaths Associated with CDI, According to Epidemiologic Category, 2011.
| Characteristic | Estimated Recurrences | Recurrence Rate | Estimated Deaths | Death Rate | ||||
|---|---|---|---|---|---|---|---|---|
| CA CDI | HCA CDI | CA CDI | HCA CDI | CA CDI | HCA CDI | CA CDI | HCA CDI | |
| no. (95% CI) | no. per 100,000 persons (95% CI) | no. (95% CI) | no. per 100,000 persons (95% CI) | |||||
| All cases | 21,600 (16,900–26,300) | 61,400 (40,200–82,600) | 7.0 (5.5–8.6) | 19.9 (13.0–26.9) | 2000 (1200–2800) | 27,300 (15,300–39,300) | 0.7 (0.4–0.9) | 8.9 (5.0–12.8) |
| Sex | ||||||||
| Male | 7800 (5100–10,500) | 27,300 (12,800–41,800) | 5.2 (3.4–6.9) | 18.0 (8.5–27.6) | 900 (450–1350) | 12,300 (3800–20,700) | 0.6 (0.3–0.9) | 8.1 (2.5–13.7) |
| Female | 13,800 (9900–17,600) | 34,000 (18,700–49,400) | 8.8 (6.3–11.3) | 21.7 (12.0–31.6) | 1100 (400–1700) | 15,000 (6600–23,500) | 0.7 (0.3–1.1) | 9.6 (4.2–15.0) |
| Age group | ||||||||
| 1–17 yr | 1400 (900–1900) | 300 (100–500) | 2.0 (1.3–2.7) | 0.4 (0.1–0.7) | NA | NA | NA | NA |
| 18–44 yr | 2600 (1300–3900) | 3400 (1000–5700) | 2.3 (1.1–3.4) | 3.0 (0.9–5.0) | 50 (0–120) | NA | <0.1 (0–0.1) | NA |
| 45–64 yr | 6200 (4000–8300) | 9000 (4400–13,700) | 7.5 (4.8–10.0) | 10.9 (5.3–16.6) | 420 (120–720) | 4500 (1020–8000) | 0.5 (0.1–0.9) | 5.4 (1.2–9.7) |
| ≥65 yr | 11,400 (7400–15,400) | 48,700 (28,100–69,200) | 27.5 (17.9–37.2) | 117.6 (67.9–167.2) | 1500 (750–2200) | 22,800 (11,300–34,200) | 3.6 (1.8–5.3) | 55.1 (27.3–82.6) |
| Race | ||||||||
| White | 19,600 (14,900–24,200) | 54,900 (34,000–75,700) | 8.1 (6.2–10.1) | 22.8 (14.1–31.5) | 1800 (980–2600) | 25,700 (13,900–37,600) | 0.8 (0.4–1.1) | 10.7 (5.8–15.6) |
| Nonwhite | 2000 (900–3200) | 6500 (400–12,600) | 3.0 (1.3–4.8) | 9.7 (0.6–18.8) | 200 (0–390) | 1600 (0–3500) | 0.3 (0.0–0.6) | 2.4 (0.0–5.2) |
A recurrence was defined as a positive result on testing for C. difficile in a stool specimen during the period from 14 days through 56 days after the initial episode of C. difficile infection (CDI). Death from CDI was defined as any death occurring within 30 days after positive results on testing for C. difficile in a stool specimen. CA denotes community-associated, HCA health care–associated, and NA not applicable because no deaths within 30 days were observed.
isolate characterization
C. difficile was isolated in samples obtained from 1364 of 1625 patients (83.9%) in whom stool culture was performed. The three most common strains in both community- and health care–associated cases were NAP1, NAP4, and NAP11, which represented mostly PCR ribotypes 027, 020, and 106, respectively (Table 4). The NAP1 strain was more common among health care–associated cases than among community-associated cases (30.7% vs. 18.8%, P<0.001). Among the 138 community-associated cases and 193 health care–associated cases with NAP1 strains, 12 isolates (8.7%) and 3 isolates (1.6%), respectively, were negative for binary toxin. The NAP7 strain (PCR ribotype 078) represented less than 4% of the isolates in the two groups, and all NAP7 isolates were positive for binary toxin.
Table 4. Distribution of C. difficile Strains, According to Epidemiologic Category.
| Strain | Community-Associated CDI (N = 735) | Health Care–Associated CDI (N = 629) |
|---|---|---|
| no. of cases (%) | ||
| NAP1 | 138 (18.8) | 193 (30.7) |
| NAP1-related† | 13 (1.8) | 20 (3.2) |
| NAP2 | 13 (1.8) | 10 (1.6) |
| NAP3 | 3 (0.4) | 12 (1.9) |
| NAP4 | 84 (11.4) | 65 (10.3) |
| NAP5 | 3 (0.4) | 6 (1.0) |
| NAP6 | 56 (7.6) | 27 (4.3) |
| NAP7 | 25 (3.4) | 13 (2.1) |
| NAP7-related‡ | 2 (0.3) | 2 (0.3) |
| NAP8 | 5 (0.7) | 1 (0.2) |
| NAP9 | 22 (3.0) | 9 (1.4) |
| NAP10 | 21 (2.9) | 15 (2.4) |
| NAP11 | 79 (10.7) | 63 (10.0) |
| NAP12 | 9 (1.2) | 16 (2.5) |
| Unnamed§ | 245 (33.3) | 163 (25.9) |
| Could not be typed¶ | 17 (2.3) | 14 (2.2) |
Molecular typing was performed with the use of pulsed-field gel electrophoresis (PFGE). PFGE types represented the following ribotypes on polymerase-chain-reaction assay, according to an analysis that was performed on a random sample of 35 of the most prevalent NAP (North American PFGE) types: NAP1, 027; NAP4, 020; NAP6, 002; NAP7, 078; and NAP11, 106.
This strain has characteristics of NAP1 (i.e., positive for toxins A and B and C. difficile binary toxin with a 18-bp deletion in tcdC) but does not meet the 80% cutoff for relatedness on PFGE.
This strain has characteristics of NAP7 (i.e., positive for toxins A and B and C. difficile binary toxin with a 39-bp deletion in tcdC) but does not meet the 80% cutoff for relatedness.
The strains in the unnamed category include 80 PFGE types that do not fall within NAP1 through NAP12.
DNA from these samples produced no bands on PFGE after three attempts.
DISCUSSION
We estimated that C. difficile caused approximately 453,000 incident infections and was associated with approximately 29,000 deaths in the United States in 2011 on the basis of data from active population- and laboratory-based surveillance across diverse geographic locations in the United States. Persons 65 years of age or older, whites, and females had higher incidences than their comparators. This national estimate of C. difficile infection is higher than previous U.S. estimates (240,000 to 333,000) that relied on passive surveillance, data from health care facilities in a single state, administrative data, or data from managed-care populations in a specific region.23–25 However, comparisons with previous estimates are limited by differences in definitions of C. difficile infection and in analytical methods, especially the emergence of NAAT testing.
Only an estimated 24% of cases occurred in hospital settings, leading to an estimate of approximately 107,600 hospital-onset infections nationally. This number is higher than the 80,400 cases of hospital-onset infections that were recently reported from a point-prevalence survey conducted from May 2011 through September 2011 in the 10 EIP sites with the use of similar definitions.9 A possible explanation for this difference is the uptake of molecular testing for C. difficile diagnosis by hospital laboratories during 2011.5,19
According to our estimates, nearly 345,400 cases occurred outside of hospitals, indicating that the prevention of C. difficile infection should go beyond hospital settings. Although 46.2% of those cases were community-associated and by definition had no documented inpatient health care exposure, in a recent study that used the same surveillance program and sites but included earlier years of data, 82% of patients with community-associated C. difficile infection reported during telephone interviews that they had visited outpatient health care settings, such as a doctor’s or dentist’s office, in the 12 weeks before the collection of a C. difficile–positive stool sample.11 Therefore, most patients with C. difficile infection had either inpatient or outpatient health care exposures before disease onset. Finally, our adjusted national rate of community-associated infection of 51.9 per 100,000 population is higher than the rate of 20 to 40 per 100,000 population that was reported from population-based studies outside the United States that were conducted before the introduction of NAAT.26,27 However, it is possible that some of the cases detected by NAAT represent colonization rather than true infection, given that NAAT detects the presence of the organism but not necessarily if it is disease-causing and has high sensitivity.28,29 The rate of asymptomatic colonization in nonhospitalized adults is estimated to be 2%, with a higher rate, up to 26%, in those with health care exposures.30–32
Recurrence rates for health care–associated C. difficile infection have been reported to vary from 5% to 50%, with an average of 20%.33–35 In our study, at least one recurrence of C. difficile infection occurred in approximately 21% of cases of health care–associated infection and 14% of cases of community-associated infection on the basis of repeated stool testing between 14 and 56 days after the initial C. difficile episode, leading to an estimated burden of 83,000 first recurrent infections. These numbers are worrisome, given challenges in treating recurrent infections and the ongoing risk of transmission when symptoms recur.32,36,37
C. difficile is known to cause severe disease and death.2,3 The estimated total number of deaths within 30 days after the diagnosis of C. difficile infection nationally was 29,300, and the majority of these deaths were among patients with health care–associated infection. This number equated to an observed 30-day crude case fatality rate of 9.3% for patients with health care–associated infection, a rate that is similar to that reported in studies of hospitalized patients with C. difficile infection.38–40 Since the mortality that is attributable to C. difficile infection is estimated to be approximately 50% of the crude mortality,38 the total number of deaths in our study that would be attributable to C. difficile infection is about 15,000. The three most common strains we observed in both community-associated and health care–associated infection (NAP1, NAP4, and NAP11) are similar to the strains that have been reported in other countries.41,42 The NAP7 strain has been isolated from food and food animals and represented around 4% of the isolates in our collection; this finding is consistent with the prevalence observed in England (4%), but lower than the 8% prevalence reported from a hospital survey involving 34 European countries.43–46
Our analyses have several limitations. First, the case definition relied solely on positive results on C. difficile toxin or molecular assay because diarrhea is usually poorly documented in charts and existing guidelines for laboratory practice recommend C. difficile testing only on unformed stools.47,48 It has been documented that laboratories are adopting stricter policies to reject formed stools when transitioning to NAAT.19 Second, the type of C. difficile diagnostic test that is used has implications for measured disease incidence. Several studies have shown that laboratories transitioning to NAAT are expected to observe an increase in C. difficile incidence, which may partially represent overdiagnosis of C. difficile infection owing to a highly sensitive assay that does not distinguish between colonization and disease.5,6,19,28,29 Our estimates of incidence and disease burden were based on a rate of NAAT use of 52%, which was observed across the EIP sites. Although this rate may not be representative of the rate of NAAT use in the United States, a sensitivity analysis showed how the burden estimate varies on the basis of NAAT use (Fig. S1 in the Supplementary Appendix). Third, since we collected data on rates of recurrence and death in a random sample of cases, these rates may not be representative. In addition, our study underestimates both recurrence and mortality, given that we assessed only first recurrences and deaths that were documented in the medical record. It is likely that a subset of patients had multiple recurrences or died after discharge from the hospital or nursing home. Additional limitations are discussed in the Supplementary Appendix.
Despite these limitations, our national estimates are based on a large, longitudinal, U.S. population–based surveillance for C. difficile infection and on active laboratory case finding by trained personnel. Our results also support the growing evidence that C. difficile is no longer restricted to acute care settings. Thus, in the absence of a vaccine, future efforts to prevent C. difficile will cross health care settings and focus more on appropriate antibiotic use, which has been shown to be successful in decreasing rates of C. difficile infection in England, where a multifaceted program including antimicrobial stewardship was implemented.49 The prevention of C. difficile infection is a U.S. priority, with 2020 national reduction targets being established and all hospitals participating in the Hospital Inpatient Quality Reporting Program of the Centers for Medicare and Medicaid Services, which has reported data regarding C. difficile infection to the National Healthcare Safety Network since 2013.50,51
In conclusion, on the basis of active population- and laboratory-based surveillance across 10 U.S. geographic areas, we estimated that C. difficile caused almost half a million infections in the United States in 2011. An estimated 83,000 of the patients with such infections had at least one recurrence, and approximately 29,000 died within 30 days after the initial diagnosis. Continued surveillance for C. difficile infection will be needed to monitor progress toward prevention.
Supplementary Material
Acknowledgments
We thank Joelle Nadle, Erin Garcia, and Erin Parker of the California EIP; Helen Johnston of the Colorado EIP; Carol Lyons of the Connecticut EIP; Leigh Ann Clark, Andrew Revis, Olivia Almendares, Zirka Thompson, and Wendy Baughman of the Georgia EIP; Rebecca Perlmutter of the Maryland EIP; Ruth Lyn-field of the Minnesota EIP; Nicole Kenslow of the New Mexico EIP; Rebecca Tsay and Deborah Nelson of the New York EIP; Valerie Ocampo of the Oregon EIP; Samir Hannah, L. Amanda Ingram, and Brenda Rue of the Tennessee EIP; Susan Sambol and Laurica Petrella of the Hines VA Hospital; and Ashely Paulick, Johannetsy Avillan, Kamile Rasheed, and Lydia Anderson of the CDC.
Supported by the Emerging Infections Program (EIP) Cooperative Agreement between the 10 EIP sites and the CDC.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official position of the CDC.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
POSTING PRESENTATIONS FROM MEDICAL MEETINGS ONLINE
Online posting of an audio or video recording of an oral presentation at a medical meeting, with selected slides from the presentation, is not considered prior publication. Authors should feel free to call or send e-mail to the Journal’s Editorial Offices if there are any questions about this policy.
Contributor Information
Fernanda C. Lessa, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta
Yi Mu, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta
Wendy M. Bamberg, Colorado Department of Public Health and Environment, Denver, Illinois
Zintars G. Beldavs, Oregon Health Authority, Public Health Division, Portland, Illinois
Ghinwa K. Dumyati, University of Rochester Medical Center, Rochester, NY, Illinois
John R. Dunn, Tennessee Department of Health, Nashville, Illinois
Monica M. Farley, Emory University School of Medicine, Department of Medicine, Atlanta Veterans Affairs Medical Center, Atlanta
Stacy M. Holzbauer, CDC Office of Public Health Preparedness and Response, Division of State and Local Readiness, Atlanta, Minnesota Department of Health, St. Paul, Illinois
James I. Meek, Yale School of Public Health, Connecticut Emerging Infections Program, New Haven, Illinois
Erin C. Phipps, University of New Mexico, New Mexico Emerging Infections Program, Albuquerque, Illinois
Lucy E. Wilson, Maryland Department of Health and Mental Hygiene, Baltimore, Illinois
Lisa G. Winston, Department of Medicine, University of California, San Francisco, School of Medicine, San Francisco, Illinois
Jessica A. Cohen, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta Research and Education Foundation, Atlanta
Brandi M. Limbago, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta
Scott K. Fridkin, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta
Dale N. Gerding, Department of Medicine, Loyola University Chicago Stritch School of Medicine, Maywood, Edward Hines, Jr., Veterans Affairs Hospital, Hines, Illinois
L. Clifford McDonald, Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta
references
- 1.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene–variant strain of Clostridium difficile. N Engl J Med 2005;353:2433–41. [DOI] [PubMed] [Google Scholar]
- 2.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med 2005;353:2442–9. [Erratum, N Engl J Med 2006;354:2200.] [DOI] [PubMed] [Google Scholar]
- 3.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 2010;23:529–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healthcare Cost and Utilization Project. HCUP Projections: Clostridium difficile infection 2011 to 2012. Report # 2012–01 (http://www.hcup-us.ahrq.gov/reports/projections/CDI_Regional_projections_Final.pdf). [Google Scholar]
- 5.Gould CV, Edwards JR, Cohen J, et al. Effect of nucleic acid amplification testing on population-based incidence rates of Clostridium difficile infection. Clin Infect Dis 2013;57:1304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longtin Y, Trottier S, Brochu G, et al. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis 2013;56:67–73. [DOI] [PubMed] [Google Scholar]
- 7.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012;55:216–23. [DOI] [PubMed] [Google Scholar]
- 8.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011;32:387–90. [DOI] [PubMed] [Google Scholar]
- 9.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012;55:Suppl 2:S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013;173:1359–67. [DOI] [PubMed] [Google Scholar]
- 12.Pawar D, Tsay R, Nelson DS, et al. Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect Control Hosp Epidemiol 2012;33:1107–12. [DOI] [PubMed] [Google Scholar]
- 13.Severe Clostridium difficile–associated disease in populations previously at low risk — four states, 2005. MMWR Morb Mortal Wkly Rep 2005;54:1201–5. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Emerging Infections Program — healthcare-associated infections projects (http://www.cdc.gov/hai/eip/cdiff_techinfo.html). [Google Scholar]
- 15.Lessa FC, Mu Y, Winston L, et al. Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis 2014. June 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis 2014;58:1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killgore G, Thompson A, Johnson S, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol 2008;46:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect 2008;14:1057–64. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J, Limbago B, Dumyati G, et al. Impact of changes in Clostridium difficile testing practices on stool rejection policies and C. difficile positivity rates across multiple laboratories in the United States. J Clin Microbiol 2014;52:632–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitjan DF, Little RJA. Multiple imputation for the Fatal Accident Reporting System. J R Stat Soc Ser C Appl Stat 1991; 40:13–29. [Google Scholar]
- 21.Lohr SL. Sampling: design and analysis. 2nd ed. Pacific Grove, CA: Duxbury Press, 2009. [Google Scholar]
- 22.United States Census Bureau home page (http://www.census.gov). [Google Scholar]
- 23.Kuntz JL, Johnson ES, Raebel MA, et al. Clostridium difficile infection, Colorado and the northwestern United States, 2007. Emerg Infect Dis 2012;18:960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol 2009;30: 526–33. [DOI] [PubMed] [Google Scholar]
- 25.Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays. Statistical brief no. 124. Rockville, MD: Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality, January 2012. (http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf). [PubMed] [Google Scholar]
- 26.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 2008;62:388–96. [DOI] [PubMed] [Google Scholar]
- 27.Norén T, Akerlund T, Bäck E, et al. Molecular epidemiology of hospital-associated and community-acquired Clostridium difficile infection in a Swedish county. J Clin Microbiol 2004;42:3635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect Dis 2013;13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol 2014;35:667–73. [DOI] [PubMed] [Google Scholar]
- 30.Aronsson B, Möllby R, Nord CE. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980–1982. J Infect Dis 1985;151:476–81. [DOI] [PubMed] [Google Scholar]
- 31.Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology 1981; 81:5–9. [PubMed] [Google Scholar]
- 32.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431–55. [DOI] [PubMed] [Google Scholar]
- 33.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis 2005;5:549–57. [DOI] [PubMed] [Google Scholar]
- 34.Eyre DW, Walker AS, Wyllie D, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis 2012;55: Suppl 2:S77–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197–205. [DOI] [PubMed] [Google Scholar]
- 36.Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med 2014; 9:418–23. [DOI] [PubMed] [Google Scholar]
- 37.Wayne E, Grein J, Murphy R. Evaluation of hospital readmissions following Clostridium difficile infection (CDI) and patient characteristics associated with CDI recurrence during hospital readmission. Presented at 2013 IDWeek, San Francisco, October 2–6, 2014. abstract (https://idsa.confex.com/idsa/2013/webprogram/Paper41261.html). [Google Scholar]
- 38.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013;34:588–96. [DOI] [PubMed] [Google Scholar]
- 39.Bloomfield MG, Carmichael AJ, Gkrania-Klotsas E. Mortality in Clostridium difficile infection: a prospective analysis of risk predictors. Eur J Gastroenterol Hepatol 2013;25:700–5. [DOI] [PubMed] [Google Scholar]
- 40.Hensgens MP, Goorhuis A, Dekkers OM, van Benthem BH, Kuijper EJ. All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clin Infect Dis 2013;56:1108–16. [DOI] [PubMed] [Google Scholar]
- 41.Miller M, Gravel D, Mulvey M, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis 2010;50:194–201. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012;55:1056–63. [DOI] [PubMed] [Google Scholar]
- 43.Ratnayake L, McEwen J, Henderson N, et al. Control of an outbreak of diarrhoea in a vascular surgery unit caused by a high-level clindamycin-resistant Clostridium difficile PCR ribotype 106. J Hosp Infect 2011;79:242–7. [DOI] [PubMed] [Google Scholar]
- 44.Costa MC, Reid-Smith R, Gow S, et al. Prevalence and molecular characterization of Clostridium difficile isolated from feedlot beef cattle upon arrival and mid-feeding period. BMC Vet Res 2012;8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weese JS, Rousseau J, Deckert A, Gow S, Reid-Smith RJ. Clostridium difficile and methicillin-resistant Staphylococcus aureus shedding by slaughter-age pigs. BMC Vet Res 2011;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011;377:63–73. [DOI] [PubMed] [Google Scholar]
- 47.Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014;133:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharp S, Gilligan P. 2010. A practical guidance document for the laboratory detection of toxigenic Clostridium difficile. Washington, DC: American Society for Microbiology, September 21, 2010. (http://www.asm.org/images/pdf/Clinical/clostridiumdifficile9-21.pdf). [Google Scholar]
- 49.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart — Then Focus. J Antimicrob Chemother 2012;67:Suppl 1:i51–i63. [DOI] [PubMed] [Google Scholar]
- 50.Notices: requests for comments on the proposed 2020 targets for the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination. Fed Regist 2014;79:10524 (http://www.gpo.gov/fdsys/pkg/FR-2014-02-25/pdf/2014-04069.pdf). [Google Scholar]
- 51.Rules and regulations, Medicare program: hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; hospitals’ FTE resident caps for graduate medical education payment; final rule. Fed Regist 2011;76:51476 (http://www.gpo.gov/fdsys/pkg/FR-2011-08-18/pdf/2011-19719.pdf). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



