Summary
Light is a potent circadian entraining agent. For many people, daily light exposure is fundamentally dysregulated with reduced light during the day and increased light into the late evening. This lighting schedule promotes chronic disruption to circadian physiology resulting in a myriad of impairments. Developmental changes in sleep-wake physiology suggest that such light exposure patterns may be particularly disruptive for adolescents and further compounded by lifestyle factors such as early school start times. This narrative review describes evidence that reduced light exposure during the school day delays the circadian clock, and longer exposure durations to light-emitting electronic devices in the evening suppress melatonin. While home lighting in the evening can suppress melatonin secretion and delay circadian phase, the patterning of light exposure across the day and evening can have moderating effects. Photic countermeasures may be flexibly and scalably implemented to support sleep-wake health; including manipulations of light intensity, spectra, duration and delivery modality across multiple contexts. An integrative approach addressing physiology, attitudes, and behaviors will support optimization of light-driven sleep-wake outcomes in adolescents.
Keywords: circadian rhythm, light, electric, sleep, melatonin, cortisol, therapy, adolescence
Light is the primary entraining agent synchronizing human circadian rhythms to the solar day (1). In humans, the central pacemaker in the hypothalamic suprachiasmatic nucleus (SCN) orchestrates the circadian timing of bodily processes, including sleep-wake patterns, hormone expression (melatonin, cortisol), and body temperature over the 24-h light-dark cycle (2). Melatonin rises in the evening (typically associated with sleep onset), and peaks in the early morning hours before falling to low levels during the daytime (3). In tandem, cortisol rises in the early morning (typically associated with wakefulness) and declines across the day until reaching an evening nadir (3). Among other cues, these hormonal signals synchronize the timing of molecular clocks found in the body and collectively this network of oscillators drives robust daily rhythms throughout our body.
In modern society, our exposure to sunlight is diminished such that we spend approximately 90% of our time indoors under low intensity light levels (4, 5). With the post-industrial rise of inexpensive electric lighting (6), society is increasingly exposed to electric light across the day, evening, and beyond bedtime, with patterns, intensity, and spectra of light diverging from that of the natural 24-h light-dark cycle. This pattern of daytime and evening electric light exposure may disrupt circadian rhythms and sleep patterns.
Adolescents are at heightened risk for sleep and circadian disruption related to light exposure due to physiological changes coinciding with puberty which include a shift to later sleep timing (7). Adolescents at a late pubertal stage exhibit slower accumulation of homeostatic sleep pressure across the day relative to adolescents in early pubertal stages, contributing to a sleep schedule that is delayed relative to that of adolescents in early puberty (8). Adolescents also demonstrate delays in circadian timing, which begin in pre-puberty (~9–10 years) and peak at the age of 20 years on average followed by a subsequent advance, providing a biological indicator for the onset and conclusion of adolescence, respectively (9, 10). Based on circadian principles, the delay in sleep-wake timing in adolescence could be easily explained by an age-dependent change in circadian cycle length or sensitivity to light. Unfortunately, neither explanation has been strongly supported by the literature. While initial research supported a lengthening of the endogenous circadian period during adolescence (11), other work found no differences in circadian period between adolescents and adults (12). Another mechanistic explanation would be age-dependent changes in the sensitivity of the circadian system to light. Youth entering puberty display an increased light-driven melatonin suppression across a range of illuminance levels (relative to adolescents at a later pubertal stage (13). When compared to adults, youth as a whole displayed greater melatonin suppression in response to light than their parents (14). These findings, taken together with research showing a strong melatonin suppression response to light in preschool children, suggest that sensitivity to evening light diminishes across development, making alterations in evening light sensitivity not especially unique to puberty (15). Moreover, in late- to- post-pubertal adolescents, the phase response curve to white light was comparable to that observed in a separate sample of adults using the same protocol (7, 16). Although more work is needed, findings to date cannot explain the sleep delay seen in adolescents by systematic changes in circadian period or light sensitivity.
Beyond physiological changes, a number of behavioral and contextual changes present during adolescence may contribute to the observed delays in sleep-wake timing. For example, adolescents undergo a rising sense of independence, increased engagement in social and extracurricular activities, heightened use of electronic devices, and growing academic pressures – all of which contribute to delayed bedtimes (17, 18). Late bedtimes combined with school-induced early rise times result in a sleep debt typically relieved by sleeping-in during the weekends. In turn, these late weekend rise times reduce adolescents’ sleep pressure and delay their circadian phase further reinforcing this delayed sleep-wake cycle (19, 20). This pattern fuels a cycle of ‘social jet lag’ in which this mismatch between delayed circadian timing and school schedule leaves adolescents sleepy and fatigued, particularly at the outset of the school week (21). This dysregulated sleep cycle may contribute to the emergence of mental and physical challenges in adolescents (22). Thus, improving adolescent light health may allay daytime impairment and mitigate risk for mental and physical health problems.
This narrative review summarizes the impact of electric light on adolescents, with particular reference to circadian phase response, melatonin suppression, cognitive arousal, and sleep disruption. It offers a research agenda to optimize adolescent circadian health through understanding the interactions between electric lighting parameters and behavior.
Light Sensation, Measurement, and Application
There is increasing recognition that the non-image forming effects of light drive human physiology and behavior. The intrinsically photosensitive retinal ganglion cell (ipRGC) photoreceptors are the neural substrate linking the retina to the SCN (23, 24) to control circadian rhythms as well as to brain regions that correspond to sleep, cognition, and pupil control (24). IpRGCs express the melanopsin photopigment that is maximally sensitive to ~480 nm (‘blueish-greenish’ light) (25–27), abundant in sunlight and some electric lighting, while also relaying sensory signals initiated by rods and cones that are functionally dissociable (28–31) including in the SCN (24). IpRGC activation (via excitation of melanopsin as well as rod and cone photopigments) is the most functionally relevant indicator of light’s impact on non-image forming behaviors and the best predictor of circadian responses such as melatonin suppression and circadian phase shifting (32). Notably, the peak sensitivity of melatonin suppression is to shorter wavelengths than the peak of the melanopsin photopigment (33), and so other photoreceptor contributions (such as S-cones) may be important (34, 35).
Advances in lighting technology have led to differences in the spectral power distributions of luminaires. New measures from the International Commission on Illumination (CIE) can estimate melanopsin and other photoreceptor excitations for any given light source if the spectral power distribution is known (36), but have rarely been reported in the adolescent lighting literature. Studies instead report measures weighted by the sensitivity of the image forming pathways only, such as luminance (cd/m2) or illuminance (lux), and/or commercially available specifications such as correlated color temperature/kelvin (CCT; K) (37), which are inadequate for characterizing the photopic stimulus for non-image forming vision. Although the new CIE measures do not account for higher-order retinal circuits, the incorporation of non-image forming anatomy and physiology can predict circadian responses for complex lights (38) even using standard image forming industry measures that do not take into account ipRGC activation (39).
Aside from spectral composition, light’s physical properties such as timing, intensity, and spatial distribution can powerfully affect human circadian rhythms. The circadian phase response curve to light describes the magnitude and direction of circadian phase shifts in response to the biological timing of light stimulation: Light administered before the core body temperature minimum or other markers of circadian timing (e.g., midpoint of sleep) typically delays circadian phase, whereas light administered after typically advances circadian phase (40). Practically, this means that exposures in the biological morning advance the rhythm, while exposures in the biological evening delay the rhythm (1). The phase shifting effects of light vary with the intensity and duration of the photic stimulation (41–43) where longer exposures drive larger phase shifts and increased melatonin suppression, but shorter exposures more efficiently shift circadian phase and suppress melatonin on a photon-for-photon basis. Lights of increasing intensity more strongly stimulate the circadian system, where short-wavelength light produces the most robust melatonin suppressing, phase shifting, and alerting effects (44–46). Spatially, light sensed across the retina may not be equally weighted for non-image forming purposes (47, 48) but is difficult to control in real-world environments. These physical characteristics of light intersect to dynamically stimulate the circadian system, with no single factor considered in isolation being sufficient (26).
Electric Lighting Exposure
Electric lighting is prevalent in schools, homes, workplaces and personal electronic devices. Adolescents are arguably at the greatest risk for circadian disruption by lighting relative to other developmental groups due to the discrepancy between their internal circadian timing, which promotes an extreme evening chronotype, and early school start times that promote an early rise time, even if the body is not well rested. Given that adolescents can spend much of the day indoors, daytime exposures can lack the high intensity and short wavelengths to robustly entrain the circadian system. Evening light exposures can also be of (relatively) high intensity, and long duration, with significant short-wavelength content to delay circadian phase and disrupt sleep patterns, while further promoting nighttime activities into the late evening. In subsequent paragraphs we describe this literature, though it should be noted that the conclusions drawn are limited by characterization of the photic stimuli for image forming vision, rather than for non-image forming vision.
Light Exposure in School
School comprises a substantial portion of adolescents’ schedules, ~33 hours per week in the U.S. (49). Educational lighting standards (e.g. IES RP-3–20) were developed to support perceptual vision (e.g., minimizing glare, improving visibility, providing sufficient horizontal and vertical illuminance for specific settings and tasks, incorporating and accounting for daylighting), but are not optimized for non-image forming vision and circadian needs (50, 51). Reduced short-wavelength light exposure (using orange-tinted glasses to block short-wavelength light) from rise time until the end of the school day produced delays in dim light melatonin onset (DLMO) (52, 53), demonstrating the importance of obtaining sufficient short-wavelength light during the school day.
This has implications for adolescents residing in highly structured or confined settings, including boarding schools, juvenile detention facilities, immigration detention centers, and residential treatment facilities. Juvenile detention centers were shown to have lower illuminance (124 lux v. 297 lux), fewer windows (2 v. 7), and earlier lights-on (6:07 am v. 6:54 am) and lights-off (8:42 pm v. 9:06 pm) times relative to treatment centers (54). Additionally, adolescents from juvenile detention centers reported bedtimes approximately 50 minutes earlier than their sleep onset times (55). This has implications for misalignment between the delayed circadian timing present in many adolescents and demands on sleep-wake schedule (i.e., early bed and rise times) for youth in juvenile detention centers, and resulting insomnia (54). Future experimental investigation is warranted to examine objective effects of electric light exposure patterns in structured adolescent institutional residential settings on sleep and circadian outcomes to establish the degree to which lighting interventions are needed.
Outdoor Electric Light at Night
Light at night (LAN) is a concern (56) for sleep-wake health, especially where efficient electrical grids and lighting promote year-round street lighting throughout the night in urbanized parts of the world (57). While high pressure sodium lighting is the most prevalent form of street lighting, LED street lighting use is increasing (56, 58) and may produce greater circadian or sleep disruptions due to the short-wavelength spectral peak inherent to LED technology (59): One hour of 18 lux or 27 lux white 6900 K LED light was predicted to stimulate the circadian system to a small extent (i.e., predicted 12% – 15% melatonin suppression), while metal halide (4000 K), high-pressure sodium (2050 K), and two cool white LED light sources (5200 K; 6900 K) did not suppress melatonin (60). Satellite measures of outdoor LAN were associated with increased subjective eveningness and later midpoint of sleep, after accounting for age and sex (61), and a later subjective weeknight bedtime, after controlling for sociodemographic factors in adolescents (62). These studies are limited by a lack of individual light-level readings, as nocturnal satellite readings do not correlate with individual indoor light exposure levels in adults or 11-to-13-year-old children (63, 64), except during the darkest period of night and only in youth whose parents reported that outdoor light occasionally or often influences indoor light levels (weak correlation). (63). However, satellite measures may still add value as they reflect community-level differences in LAN exposure (e.g., satellite LAN readings were correlated with increased air pollution, urbanicity, population density, reduced greenery, and lower socioeconomic status in youth) (63, 65). An expert panel recommended measuring LAN with calibrated devices whenever possible and using these data to validate questionnaires to serve as a reliable proxy for objective LAN measures in epidemiological studies (64).
Light-emitting Electronic Devices
A number of studies have evaluated the relationship between light-emitting electronic devices and adolescent sleep. Cross-sectional studies show device usage is associated with later bedtimes, longer sleep onset latency, reduced sleep duration, and increased sleep problems (66). Mobile phones may account for much of the impairment, with increased duration and later timing of use associated with poorer sleep quality in adolescents (67). However, this relationship may be mediated by several mechanisms: Using electronic devices before bed or in bed may directly displace sleep with the user remaining awake longer than without the device (68, 69). Some adolescents may engage with the device due to difficulty initiating sleep (68), and the device itself may have an alerting or physiologically arousing effect, delaying the settling process that promotes sleep onset (69). The LED screens in many electronic devices emit short-wavelength light that is known to delay circadian timing with evening exposure, and there have long-been concerns that light-emitting devices contribute to suppression of slow wave sleep in the first non-rapid eye movement cycle and delays in sleep and circadian timing (69–71). While light intensity and spectrum can both be of concern with these devices, it is likely that light intensity, rather than spectrum, is the dominant factor contributing to disruption (72) under real-world device usage.
Laboratory-controlled studies have evaluated the impact of light exposure from various electronic devices. A comparison of 1 h of evening exposure to bright (80 lux), dimmed (1 lux), and short-wavelength filtered (50 lux) tablet light exposure in adolescents showed no differences in a variety of sleep measures (73). In a separate study, exposure to 2 h (but not 1 h) of self-luminous tablet light (40 lux) at maximum brightness among adolescents and young adults was associated with increased melatonin suppression (74). These findings suggest that extended evening light exposures can inhibit the evening rise in melatonin, but the impacts on adolescent sleep are unknown.
Addressing the lack of ecological validity in laboratory studies, a home-based study in adolescents showed that viewing various self-luminous devices (average 87 lux) without orange-tinted (blue-light-blocking) glasses for 2 h on one evening was associated with increased melatonin suppression relative to viewing them through orange-tinted glasses for 3 h (75). Further, viewing self-luminous devices without orange-tinted glasses was associated with melatonin suppression following exposure for 1 and 2 h. Adolescents appear sensitive to light from electronic devices at 1 and 2 h of exposure in the home environment. However, it is unclear how device type, style of device engagement, and home lighting influenced outcomes (75).
A further home-based study in undergraduate students showed no main effects for tablet light exposure (unfiltered [< 200 lux] or short-wavelength filtered light [200 lux]) or stimulation level (participant’s Facebook account versus a sham Facebook account designed to be unstimulating), but found that the short-wavelength filtered and low stimulation condition was associated with significantly better self-reported sleep quality, sleep duration, sleep onset latency, and daytime function relative to the other conditions (76). The pattern of findings suggests that engaging in stimulating tasks while using electronic devices before bed disrupts sleep regardless of spectrum, but only the modification of stimulation level and light spectrum in tandem is associated with improved subjective sleep and daytime functioning.
Home Lighting
Although there has been minimal investigation of home lighting exposure among adolescents, studies in rural populations with and without electric home lighting are informative. Adolescents in rural Brazil with electric home lighting showed reduced sleep duration on school days relative to weekends and across the week, later sleep onset on school days relative to adolescents without electric home lighting (77, 78). However, the duration of evening light exposure is an important consideration. As a benchmark, 30 minutes of exposure to 30 lux is suggested as the threshold at which light begins to stimulate the circadian system (predicting 15% melatonin suppression) for incandescent lighting exposure in the home among women (79). In young adults, brighter home lighting was associated with later DLMO relative to dim home lighting, indicating the circadian phase-delaying influence of increased light intensity (80). Laboratory-controlled research corroborates these findings, indicating exposure to 3 h of fluorescent evening bright light (2000 lux) suppressed the rise in melatonin, whereas exposure to 3 h of dim light (60 lux) did not (81).
Wearable spectrometers indicate melanopic illuminance in the 3 h prior to bedtime can vary more than 10-fold (3.9–77.4 melanopic lux) across homes (82). The melanopic illuminance required for 50% melatonin suppression ranged from 3.1 to 181 melanopic lux, suggesting a high degree of inter-individual variability in sensitivity to any single home lighting setting (83). Relative to sunset, which showed rapid decreases from 181 to 3.1 melanopic lux within 22 minutes, the melanopic content of home lighting persisted through the evening, with an average of 13 melanopic lux observed at 10:00 pm, suggesting home lighting extends sunset in terms of its impact on the circadian system. Higher melanopic illuminance within the 3 h before bedtime was associated with increased wakefulness in the 90 min following bedtime. Higher melanopic illuminance was measured in homes with fluorescent and LED lighting, relative to incandescent lighting, suggesting that newer lighting technologies can negatively impact sleep and circadian health (83).
This is also relevant for sleeping environments within institutional residential facilities. Fifty-eight percent of youth across multiple juvenile detention facilities reported partial or overhead lights were turned on in spaces designated for sleep, and 23% endorsed the presence of “other” types of lighting, with the majority of those “other” sources being ‘blue’ light. Youth reported night awakenings lasting 16.8 minutes, and sleep quality and difficulty rising in the average range (55). Although descriptive, findings suggest the presence of sleep- and circadian-disrupting lighting practices in juvenile detention facilities; and these may extend to other types of institutional residential facilities.
Light Exposure across the Day and Evening
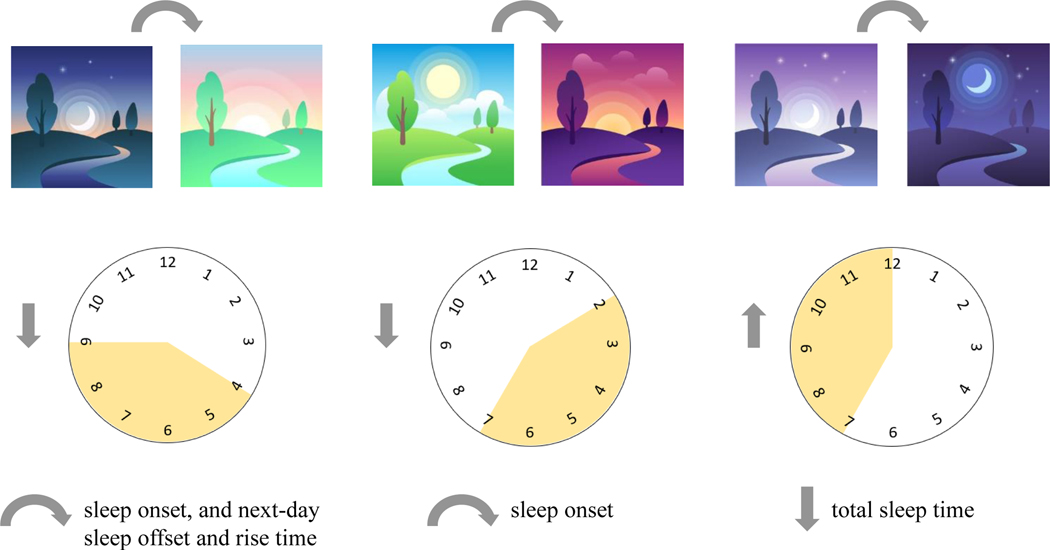
Beyond the immediate impact of light exposure delivered in specific contexts, cumulative light exposure history is important: increased daytime light exposure is associated with increased evening melatonin production and advanced circadian phase (84, 85). This may in part be due to daytime light reducing subsequent sensitivity to evening light (86, 87). In a small (n = 15) sample of adolescents, higher prior-day mean light intensity predicted earlier sleep onset but not offset, and longer sleep duration (88). A bidirectional association was found, with later prior-night sleep offset predicting lower mean light intensity. Similarly, evening chronotype adolescents with lower intensity light exposures across the preceding 24 h had later sleep onset and offset. Later last-exposure >10 lux of light associated with later sleep onset and bedtime and a reduction in total sleep time (89). Reduced morning light was associated with later sleep onset, next-day offset, and rise time (see Figure 1). Decreased afternoon-to-early-evening light was associated with later sleep onset, and increased evening-to-late-night light was associated with decreased total sleep time (89).
Figure 1.
The Relationship between Light Exposure Timing and Sleep in Adolescents (1). Decreased light from 4:00–9:00 a.m. is associated with delayed sleep onset, and next-day sleep offset and rise time. Decreased light from 2:00–7:00 pm is associated with delayed sleep onset per actigraphy. Increased light exposure between 7:00 pm–12:00 am is associated with decreased total sleep time (1).
In a case-control study over 2 weeks, adolescents with delayed sleep-wake phase disorder (DSWPD) received less morning light and more evening light relative to controls. Interestingly, evening light levels (8:00 pm to 5:00 am) were relatively dim (<48 lux) across both groups and all week days (school and non-school), with less morning light on school relative to non-school days. As adolescents slept in dim light, above findings suggest light exposure patterns among groups were well aligned with group differences in sleep schedule, and school versus non-school days (90). When data were reanalyzed relative to sleep onset rather than clock time, adolescents with DSWPD instead had less pre-sleep light exposure in the 9 h prior to sleep onset than controls, and showed no difference in light exposure in the 9 h post-sleep offset. The authors suggest increased evening light may not drive phase delays in adolescents with DSWPD, and instead reduced sensitivity to the advancing effects of morning light exposure may contribute (90). As individuals with DSWPD have longer circadian periods, resulting in a greater tendency to delay circadian rhythms relative to controls, the combination of decreased evening light and comparable morning light may serve to stabilize their rhythm, although at a later clock time (91).
These studies show that, especially for circadian disorders, individual differences in circadian physiology and activity preferences can reveal information otherwise obscured when data are referenced only to solar time. Further, it is important to understand the compounding effects of photic history over time. While strong daytime light exposures are protective against night time light exposures, day-to-day reinforcement of circadian amplitude would result in improved circadian amplitudes and robustness to perturbation by evening light. The temporal kinetics of these processes have yet to be determined in health or disease, but will better explain how photic history can attenuate (or facilitate) the acute effects of lighting.
Electric Lighting Interventions and Corrective Agents
Modern light therapy (LT) originated in the 1980s to combat depressed mood in seasonal affective disorder (92) and has been applied to an array of sleep and psychiatric disorders. LT traditionally involved remaining seated for 30 min to 1 h in front of a “light box” emitting white light of 2,500 to 10,000 lux (93). LT is typically administered just after waking to advance circadian phase, and just before bedtime to delay circadian phase (93). Morning LT is typically administered daily until reaching the desired sleep-wake schedule. As weekday morning use may conflict with school schedules, an alternative is weekend-only LT to counteract circadian delays. While one hour of LT at natural weekend risetime was not effective in mitigating the typical circadian phase delay (19), weekend LT administered 1 h after the weekend midpoint of sleep advanced adolescents’ circadian phase, with a greater effect still with 2.5 h of light (94). These findings underscore the importance of administering LT within the optimal time window per the phase response curve.
Short-wavelength light exposure is associated with increased melatonin suppression relative to long-wavelength light exposure in adolescents and adults (95–97), and increased morning cortisol relative to dim light (98). Short-wavelength LT is increasingly being utilized. The “light box” itself may also pose treatment barriers as the need to sit in front of the device for long durations may decrease adherence (93). One alternative is wearable glasses-like or visor-like devices (99, 100), which provide light therapy while the user remains mobile to engage in other activities. Three weeks of morning wearable, white LT resulted in improvements in sleep onset, sleep quality, and daytime sleepiness in adolescents with DSWPD relative to orange-light placebo (99), and sleep outcomes align with those of the light box (101). Wearable, short-wavelength LT has been shown to effectively phase advance the melatonin circadian rhythm in young adults (102), and has been investigated in adolescents, whose findings are later detailed.
While the dominant LT paradigm has entailed long duration light exposures, emerging studies demonstrate that the circadian system is also sensitive to pulse trains of millisecond-scale flashes. Such stimuli have been applied in the biological night (either during sleep or awakened to view the stimuli) and can produce larger phase shifts than continuous light exposure despite orders of magnitude fewer photons (30, 31, 41). Importantly, exposure to this flashed light during the night does not increase arousal, unlike continuous light, and does not acutely suppress melatonin. The temporal properties of the stimuli appear critical; 2 ms (41) and 2 s (31) flash durations can produce significant acute melatonin suppression, while the phase shift response is highly dependent on the inter-stimulus interval between flashes (41). Flashed light paradigms likely entail strong drive to the cone or rod photoreceptor inputs to ipRGCs that reach the SCN while simultaneously minimizing the sensory adaptation occurring with continuous light. In an in-home trial with adolescents, pulsed LT applied during the night improved sleep timing only when paired with cognitive-behavioral therapy (CBT) (103). Interventions are therefore most effective when both physiology and psychology are supported.
Supplementing Light Therapy
Several studies have examined the influence of supplementing morning LT with adjunctive interventions. Morning physical activity is known to advance circadian phase, but its potential for optimizing LT is still unclear (104). Morning short-wavelength (‘blueish-greenish’) or long-wavelength (‘redish’) LT with morning physical activity or sedentary activity in adolescents and young adults with DSWPD produced no significant group differences in improvements in sleep timing, sleep onset latency, and daytime functioning (104). However, the lack of objective increase in physical activity in the expected groups, and inclusion of three weekly sessions centered on advancing sleep-wake schedule across groups limits identification of the drivers of noted improvements (104).
Exogenous melatonin, which has soporific effects in some when administered 30–60 min prior to sleep, and circadian phase advancing or delaying effects when administered in the early evening or early morning hours, respectively, has also been investigated as an adjunctive treatment (105). However, 2 weeks of bright or dim LT with melatonin or placebo, along with gradual advancement of rise times across these four groups in adolescents and young adults yielded no significant group differences in improvements in DLMO, sleep disturbance, sleepiness and fatigue in all groups (106, 107). Three additional months of combined bright LT and melatonin were associated with greater reductions in sleep disturbance, sleepiness, and fatigue relative to no additional treatment (106, 107), suggesting the benefit of longer treatment duration.
A few studies have investigated LT with CBT (i.e., psychoeducation, cognitive restructuring, motivational interviewing, management of daytime effects of sleep loss, and relapse prevention) for youth with DSWPD or delayed sleep schedule (103, 108, 109). When compared to waitlist-control, LT with CBT showed preliminary efficacy in improving sleep onset latency, sleep onset time, total sleep time on school nights, wake after sleep onset, rise time, daytime sleepiness, and fatigue (108). However, relative to LT alone, LT with CBT was associated only with greater reductions in severity of sleep disturbance (109). The authors postulated that basic sleep education provided to both groups at treatment outset may have washed out CBT effects (109). A two-phase trial in adolescents addressed this research question from multiple perspectives. Results showed that pulsed LT produced greater improvements in sleep efficiency relative to sham-control, whereas pulsed LT with CBT was associated with improvements in a number of sleep measures relative to sham-control with CBT (103). These studies suggest LT alone may not be sufficient to produce significant change in sleep and circadian timing in adolescents. In contrast, LT with CBT produces more robust effects on sleep timing and measures of sleep disturbance in adolescents relative to waitlist-control and CBT alone, but the degree to which this combined intervention improves upon LT alone may require direct comparison in future studies. CBT provides additional structure and motivational enhancement to LT, which have utility for adolescent behavioral change.
Indeed, motivational components of increased desire and commitment to change are associated with greater adherence to advancing rise time schedule among adolescents and young adults receiving wearable, short-wavelength LT (110). Beyond individually-delivered CBT, school-based sleep education programs (SEP) provide wider treatment dissemination with limited provider involvement. Such SEP could also be administered to adolescents as part of institutional residential facility (e.g., boarding schools, treatment center, juvenile detention center) programming. Employing a motivational framework within SEP increased knowledge and motivation, but not sleep or daytime sleepiness relative to a control intervention (82). Efforts to supplement SEP with one weekend of wearable, short-wavelength LT did not bolster treatment effects on sleep knowledge, sleep outcomes, or motivation to engage in treatment components relative to SEP with parental involvement, SEP with parental involvement and LT, and class-as-usual; although a low dose of the adjunctive interventions may have contributed to the lack of group differences (111).
Interventions and Corrective Agents to Minimize Evening Light Exposure
An additional consideration is lighting interventions and corrective agents minimizing evening light exposure. University students exposed to one month of evening incandescent light exposure with a tryptophan and vitamin B6-rich breakfast (to stimulate synthesis of serotonin in the day, a melatonin precursor, and subsequent melatonin synthesis in the evening) followed by morning sunlight, had higher melatonin concentration relative to those receiving breakfast with sunlight alone, and no intervention (112). Additionally, 10 nights of 1-hour of pre-bedtime exposure to a low CCT (2000 K) 160 lux white LED lighting intervention administered to adolescents within the natural bedroom environment was associated with increased subjective sleep quality and decreased morning sleepiness and fatigue in response to 15 minutes of exercise relative to a high CCT (6000 K) 160 lux fluorescent lamp (113). Together, these findings suggest the importance of exposure to evening light of low CCT when other lighting factors are equal. Blue-light-blocking glasses, with amber or orange lenses, are another option (114). Two weeks of evening use from 9:00 pm until bedtime was associated with earlier sleep onset time, but no significant advance in DLMO in adolescents and young adults with DSWPD (115). Adolescents and young adults with sleep disturbance or irregularity showed no significant differences in sleep duration or night awakenings with a one-week course of blue light blocking glasses relative to non-blue-light-blocking glasses (116). In adolescent males, one week of evening use of blue-light-blocking glasses while engaged in device use was associated with significantly less evening melatonin suppression, and reduced vigilant attention and self-reported alertness before bedtime, but no differences in EEG-based or subjective sleep relative to clear glasses (117). Given the lack of associated adverse effects and ease of use over the short-term, blue-light-blocking glasses warrant further investigation to clarify the extent of their benefits, and establish optimal wear time, and long-term feasibility.
Shifting screens to warmer colors (e.g., “night shift mode”) to reduce short-wavelength light exposure is becoming a common feature on personal devices. In young adults, cool (5997 K) and warm (2837 K) CCT modes viewed through a commercial tablet on full brightness (97.8 lux and 54.3 lux, respectively) from a distance of 12 in. both suppressed melatonin less than short-wavelength light-emitting (40 lux of 470 nm light) goggles, but with no effect of CCT mode. This suggests a limited benefit of shifting CCT for preserving evening melatonin secretion without also decreasing illuminance (118). With respect to sleep effects, there were no differences in sleep patterns among 18–24-year-old young adults using phones set to the lowest brightness, with night shift mode enabled on the warmest color setting, no night shift mode, or no phone use for 1 hour prior to bed over 7 days in their home setting. However, for young adults with ≥ 6.8 hours of sleep per night over the week, no phone use was associated with higher sleep efficiency and decreased wake after sleep onset relative to night shift use. Surprisingly, phone use without night shift mode was associated with decreased wake after sleep onset relative to night shift mode use; suggesting that using night shift mode is not advantageous for sleep relative to no night shift mode, nor comparable to no phone use (119). Together, these studies suggest that reducing screen brightness or refraining from light-emitting device use in the hour before bed outweigh the effects of warm color-shifted devices.
Circadian-adapted School Lighting Interventions
The school environment provides a significant opportunity to stimulate the circadian system in adolescents. However, only a few studies have investigated the effects of LED or short-wavelength-enriched lighting in school settings or laboratory-based settings which mirror the classroom. There was no effect of LED (4000 K) relative to fluorescent (4000 K) school lighting type on afternoon cortisol suppression over six months (November to April; winter to spring) in 16–17-year-old students. However, the LED group showed increased afternoon cortisol suppression during darker months (i.e., November). Nevertheless, from the lack of lighting differences in cortisol suppression during brighter months, authors surmised that natural daylight conditions may have more strongly influenced cortisol suppression than the light sources (120). Additionally, morning exposure to short-wavelength-enriched white LED classroom lighting (5500 K, with additional LED modules 14000 K; 300 lux; direct/indirect luminaire) was associated with improvements in processing speed and concentration relative to fluorescent lighting (3000 K and 3500 K; ~300 lux; direct luminaire) in high school students. Unfortunately, only a subsample completed sleep diaries, and among them, there was no effect of lighting system (121).
Despite the potential of circadian-adapted (short-wavelength-enriched) lighting systems to improve daytime functioning among students, there are concerns regarding possible adverse effects (e.g., overstimulation, melatonin suppression in the afternoon hours). This has spurred interest in dynamic lighting systems which alter CCT across the day (122). In a laboratory-controlled experiment, exposure to 10 min of dynamic LED lighting (6500 K short-wavelength-enriched white light to 5000 K neutral white light; illuminance at 800–1000 lux) was associated with increased objective focus and arousal relative to 10 min of constant neutral white light (5000 K at 800–1000 lux) and 10 min of lights-off (< 1 lux) in high school students (122). While some LED lighting produces alerting effects, this is dependent on the luminaire’s design, and laboratory-controlled research needs to determine how the physical parameters of the light (e.g., spectrum, intensity) can be optimized by time of day to enhance circadian entrainment and improve sleep patterns.
A separate laboratory-controlled study showed that morning short-wavelength (‘blue-ish’)-enriched fluorescent lighting (peak at 458 nm; 1063 lux) was associated with enhanced attention on school-like tasks relative to morning long-wavelength (‘red-ish’)-enriched fluorescent lighting (peak at 611 nm; 876 lux); and circadian-consistent lighting (i.e., 1 h of short-wavelength-enriched morning light and 1 h of long-wavelength-enriched evening light) was associated with non-significant trends toward decreased nighttime awakenings. A subsample who received later evening light exposure trended towards reduced sleep onset latency relative to circadian-contrasting lighting (i.e., morning long-wavelength-enriched light and evening short-wavelength-enriched light). Thus, lighting environments during the school day and at home in the evening might improve sleep when they mimic the naturalistic changes in light that occur across the solar day and evening (123). Aforementioned adolescent institutional residential facilities could provide a structured environment through which to test the effects of such lighting regimens on sleep and circadian timing.
Some studies are limited by the measurements reported, as spectral power distribution may differ for light of the same CCT, impacting the light source’s effects on the circadian system. Further, classroom lighting simulations indicate that luminaires with a high vertical illuminance to horizontal illuminance ratio are recommended for overhead classroom lighting to better impact the circadian system, and illuminance has a greater impact on circadian stimulus than CCT (K). Guidelines recommend maximizing energy-efficiency of circadian-adapted classroom lighting through use of supplemental short-wavelength light, luminaires with high vertical-to-horizontal illuminance, and the strategic use of direct and indirect luminaires (124).
Conclusion
Adolescence is marked by delays in the timing of sleep which are likely explained by a combination of physiological (i.e., slower accumulation of homeostatic sleep drive, delaying of circadian phase) and contextual-behavioral (e.g., homework burden, extracurricular activities, social interests and pressures) factors that contribute to increased evening light exposure. The principal drivers of these changes in sleep timing are not known and developing a mechanistic understanding is important for designing optimal interventions that support sleep-wake health in adolescents.
Much emphasis has been placed on examining the relation between light emitted by electronic devices as a source of sleep and circadian disruption among adolescents. Despite a tenuous relationship found, particularly at shorter exposures, there are a number of other facets of device use (e.g., timing, intensity, viewing distance, stimulation level of activity, contrast with daylight lighting) which may intersect to impact sleep and circadian rhythms. These characteristics require further investigation in in situ experiments with a special emphasis on the effects of home lighting.
An important question is whether adolescents collectively display unique light exposure patterns relative to other age groups. New technologies such as wearable light spectrometers capture the complexities of light exposure throughout the day and night. Understanding the behavioral (e.g., physical activity, social and school engagement, disruptive behavior), contextual (e.g., school schedule, family environment, neighborhood, culture), and emotional (e.g., depression, anxiety) predictors of light exposure patterns among adolescents is an important area for future work. With this knowledge, the hope is that tailored interventions can be applied to correct the common sleep-related problems and daytime impairments observed in this age group.
Despite uncertainties regarding adolescent differences in light sensitivity and exposure, delayed circadian phase is a hallmark of adolescence (7). While morning LT is confirmed to correct circadian phase delay, morning LT in isolation may not substantially advance circadian phase and improve sleep in adolescents. LT protocols have included overt adjunctive components (e.g., CBT, motivational interviewing, supplemental melatonin, physical activity) or behavioral components (i.e., sleep hygiene, advancing of sleep-wake schedule), which cloud the ability to parse the unique contributions of each element to treatment response. Component analysis would support the identification and prioritization of interventions that maximize motivation, treatment engagement, and therapeutic outcomes in this group. Ambient lighting systems may also address adolescent motivational issues by providing a lower-effort LT delivery modality. Schools (e.g., first classroom of the day) and institutional settings provide one such means of administering LT, should future research indicate that short-wavelength-enriched lighting improves sleep and circadian timing in these settings. Home-based interventions perhaps hold more immediate relevance, and among them, dynamic home lighting (125), or better still, tunable home lighting personalized to individual circadian needs and light sensitivities (126) may be most effective.
LED lighting is expected to dominate both institutional and home settings in the immediate future (127) with the capacity for high intensity and precision temporal and spectral tuning. While humancentric or circadian lighting is already being marketed to consumers, in many cases the science does not support the manufacturer claims (72). Additionally, there are significant inter-individual differences in non-image forming light sensitivities (128) and these differences may eclipse the relatively minor modulations to the physical properties of light that have been studied (30). Understanding the source(s) of this variability in light sensitivity, and any interactions with adolescence, is necessary for moving towards personalized and powerful lighting interventions (126).
Practice Points.
Adolescents display physiological (circadian phase delay, slower sleep debt accumulation) and contextual changes which may enable increased evening light exposure in association with delayed bedtimes.
Longer durations of exposure to light from electronic devices inhibit evening rise in melatonin, but there is not yet clear evidence that the dim light emitted from electronic devices causes the delay in sleep onset seen in this age group.
Morning LT alone may not be sufficient to improve sleep and circadian timing in adolescents. CBT may boost therapeutic response to LT.
Modifications to light exposure across settings are likely to be more successful the closer they recapitulate the solar day (i.e., bright days and dark nights).
Research Agenda.
Future research should:
Understand the extent to which physiological changes relative to behavioral and contextual factors contribute to the delays in sleep-wake timing observed during adolescence.
Evaluate developmental differences in light exposure patterns using calibrated wearable spectrophotometers, and pending differences, investigate the degree to which prescribed changes to light exposure among adolescents improve social jetlag and daytime functioning.
Identify behavioral, contextual, and emotional predictors of light health.
Perform component analysis to ascertain the active elements of multi-component LT interventions.
Investigate the impact of circadian-adapted continuous or dynamic lighting interventions on sleep and circadian outcomes across multiple settings.
Better quantify lighting devices using emerging metrics and light spectral power distribution in published reports.
Acknowledgments
This review was supported by the National Institute of Mental Health K23MH113884 grant funding to Dr. Ricketts, and an American Academy of Sleep Medicine Foundation Focused Projects Grant for Junior Investigators Award (#255-FP-21), Brain and Behavior Research Foundation Young Investigator Grant (#28056), Knights Templar Eye Foundation Career Starter Grant, and National Academy of Medicine Healthy Longevity Global Catalyst Award (#2000012740) to Dr. Joyce. The content is the responsibility of the authors and is not necessarily representative of the views of the grant funding agencies.
Abbreviations:
- LAN
light at night
- CBT
cognitive-behavioral therapy
- CCT
correlated color temperature
- CIE
International Commission on Illumination
- DLMO
dim light melatonin onset
- DSWPD
delayed sleep-wake phase disorder
- ipRGC
intrinsically photosensitive retinal ganglion cell
- K
kelvin
- LED
light emitting diode
- LT
light therapy
- SCN
suprachiasmatic nucleus
- SEP
sleep education program
Footnotes
Conflicts of Interest
Dr. Burgess serves on the scientific advisory board for Natrol, LLC, and Moving Mindz, Pty Ltd, and is a consultant for F. Hoffmann-La Roche Ltd. Dr. Colwell serves as a consultant for RealSleep™. Dr. Lack receives royalties from Re-Timer Pty Ltd, and is a shareholder in the company. The other authors have no conflicts of interest to disclose.
References
An asterisk denotes the most important references.
- 1.Foster RG, Hughes S, Peirson SN. Circadian Photoentrainment in Mice and Humans. Biology. 2020;9(7):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. Journal of Sleep Research. 2016;25(2):131–43.* [DOI] [PubMed] [Google Scholar]
- 3.Selmaoui B, Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sciences. 2003;73(26):3339–49. [DOI] [PubMed] [Google Scholar]
- 4.Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It’s about time: A comparison of Canadian and American time–activity patterns. Journal of Exposure Science & Environmental Epidemiology. 2002;12(6):427–32. [DOI] [PubMed] [Google Scholar]
- 5.Fournier C, Wirz-Justice A. Light, health and wellbeing: Implications from chronobiology for architectural design. . World Health Design: Architecture, Culture, Technology. 2010;3(3):44–9. [Google Scholar]
- 6.Powell AL. Today’s Trends in Lighting. Transactions of the American Institute of Electrical Engineers. 1936;55(10):1100–10. [Google Scholar]
- 7.Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: New evidence informing the perfect storm model. Journal of Adolescence. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenni OG, Achermann P, Carskadon MA. Homeostatic Sleep Regulation in Adolescents. Sleep. 2005;28(11):1446–54.* [DOI] [PubMed] [Google Scholar]
- 9.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Current Biology. 2004;14(24):R1038–R9.* [DOI] [PubMed] [Google Scholar]
- 10.Randler C, Faßl C, Kalb N. From Lark to Owl: developmental changes in morningness-eveningness from new-borns to early adulthood. Scientific Reports. 2017;7(1):45874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neuroscience Letters. 1999;260(2):129–32. [DOI] [PubMed] [Google Scholar]
- 12.Crowley SJ, Eastman CI. Free-running circadian period in adolescents and adults. Journal of sleep research. 2018;27(5):e12678-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J Clin Endocrinol Metab. 2015;100(11):4067–73.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi S, Nagafuchi Y, Lee S-i, Harada T. Influence of Light at Night on Melatonin Suppression in Children. The Journal of Clinical Endocrinology & Metabolism. 2014;99(9):3298–303. [DOI] [PubMed] [Google Scholar]
- 15.Akacem LD, Wright KP Jr., LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Rep. 2018;6(5):e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley SJ, Eastman CI. Human Adolescent Phase Response Curves to Bright White Light. J Biol Rhythms. 2017;32(4):334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. [DOI] [PubMed] [Google Scholar]
- 18.Gaarde J, Hoyt LT, Ozer EJ, Maslowsky J, Deardorff J, Kyauk CK. So Much to Do Before I Sleep: Investigating Adolescent-Perceived Barriers and Facilitators to Sleep. Youth & Society. 2020;52(4):592–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiology International. 2010;27(7):1469–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep and Biological Rhythms. 2008;6(3):172–9. [Google Scholar]
- 21.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiology International. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 22.Touitou Y Adolescent sleep misalignment: a chronic jet lag and a matter of public health. Journal of Physiology-Paris. 2013;107(4):323–6. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt TM, Do MTH, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-Positive Intrinsically Photosensitive Retinal Ganglion Cells: From Form to Function. The Journal of Neuroscience. 2011;31(45):16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berson DM, Dunn FA, Takao M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science. 2002;295(5557):1070–3.* [DOI] [PubMed] [Google Scholar]
- 25.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280(1759):20122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Research Reviews. 2005;50(2):213–28.* [DOI] [PubMed] [Google Scholar]
- 27.Kelbsch C, Strasser T, Chen Y, Feigl B, Gamlin PD, Kardon R, et al. Standards in Pupillography. Frontiers in Neurology. 2019;10(129). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyce DS, Feigl B, Cao D, Zele AJ. Temporal characteristics of melanopsin inputs to the human pupil light reflex. Vision Research. 2015;107:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joyce DS, Feigl B, Zele AJ. The Effects of Short-Term Light Adaptation on the Human Post-Illumination Pupil Response. Investigative Ophthalmology & Visual Science. 2016;57(13):5672–80. [DOI] [PubMed] [Google Scholar]
- 30.Joyce DS, Spitschan M, Zeitzer JM. Integration of brief light flashes varying in intensity and duration by the human circadian system. bioRxiv. 2020:759134. [Google Scholar]
- 31.Figueiro MG, Bierman A, Rea MS. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat Sci Sleep. 2013;5:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TM. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. Journal of Pineal Research. 2020;69(1):e12655.* [DOI] [PubMed] [Google Scholar]
- 33.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor. The Journal of Neuroscience. 2001;21(16):6405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown TM, Thapan K, Arendt J, Revell VL, Skene DJ. S-cone contribution to the acute melatonin suppression response in humans. Journal of Pineal Research. 2021;71(1):e12719. [DOI] [PubMed] [Google Scholar]
- 35.Spitschan M, Lazar R, Yetik E, Cajochen C. No evidence for an S cone contribution to acute neuroendocrine and alerting responses to light. Current Biology. 2019;29(24):R1297–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlangen L CIE international Standard (CIE S 026/E:2018) “System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light” http://www.cie.co.at/publications/international-standards. 2018. [Google Scholar]
- 37.Kruisselbrink T, Dangol R, Rosemann A. Photometric measurements of lighting quality: An overview. Building and Environment. 2018;138:42–52. [Google Scholar]
- 38.Rea MS, Nagare R, Figueiro MG. Modeling circadian phototransduction: retinal neurophysiology and neuroanatomy. Frontiers in Neuroscience. 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong W, Zandi B, Trinh VQ, Khanh TQ. Circadian metric – Computation of circadian stimulus using illuminance, correlated colour temperature and colour rendering index. Building and Environment. 2020;184:107146. [Google Scholar]
- 40.Joyce DS, Zeitzer JM. Circadian Rhythms. Management of Sleep Disorders in Psychiatry. In: Chopra A, Das P, Doghramji K, editors. Oxford, UK: Oxford University Press; 2020. [Google Scholar]
- 41.Najjar RP, Zeitzer JM. Temporal integration of light flashes by the human circadian system. The Journal of Clinical Investigation. 2016;126(3):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang A-M, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, et al. Human responses to bright light of different durations. The Journal of Physiology. 2012;590(13):3103–12.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner PL, Van Someren EJW, Mainster MA. The role of environmental light in sleep and health: Effects of ocular aging and cataract surgery. Sleep Medicine Reviews. 2010;14(4):269–80. [DOI] [PubMed] [Google Scholar]
- 44.Lockley SW, Brainard GC, Czeisler CA. High Sensitivity of the Human Circadian Melatonin Rhythm to Resetting by Short Wavelength Light. The Journal of Clinical Endocrinology & Metabolism. 2003;88(9):4502–5. [DOI] [PubMed] [Google Scholar]
- 45.Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005;20(3):270–2. [DOI] [PubMed] [Google Scholar]
- 46.Revell VL, Arendt J, Fogg LF, Skene DJ. Alerting effects of light are sensitive to very short wavelengths. Neuroscience Letters. 2006;399(1):96–100. [DOI] [PubMed] [Google Scholar]
- 47.Nagare R, Rea MS, Figueiro MG. Spatial sensitivity of human circadian response: Melatonin suppression from on-axis and off-axis light exposures. Neurobiology of Sleep and Circadian Rhythms. 2021;11:100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glickman G, Hanifin JP, Rollag MD, Wang J, Cooper H, Brainard GC. Inferior Retinal Light Exposure Is More Effective than Superior Retinal Exposure in Suppressing Melatonin in Humans. Journal of Biological Rhythms. 2003;18(1):71–9. [DOI] [PubMed] [Google Scholar]
- 49.Juster T, Ono H, Stafford F. Changing times of American youth: 1981–2003. Institute for Social Research, University of Michigan. [Google Scholar]
- 50.IES Standards Committee. ANSI/IES RP-3–20 Recommended practice: Lighting educational facilities: An American national standard. Illuminating Engineering Society, [Internet]. 2020. Available from: www.ies.org. [Google Scholar]
- 51.Safranek S, Collier JM, Wilkerson A, Davis RG. Energy impact of human health and wellness lighting recommendations for office and classroom applications. Energy and Buildings. 2020;226:110365. [Google Scholar]
- 52.Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinol Lett. 2010;31(1):92–6. [PMC free article] [PubMed] [Google Scholar]
- 53.Figueiro M, Brons J, Plitnick B, Donlan B, Leslie R, Rea M. Measuring circadian light and its impact on adolescents. Light Res Technol. 2011;43(2):201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adornetti J, Carlucci M, Crowley S, Fleshman C, Jobe S, Wolfson A. 0979 Observational Analysis Of Juvenile Justice Sleep-wake Environment. Sleep. 2020;43:A372. [Google Scholar]
- 55.Woodard K, Adornetti J, Nogales JM, Foster M, Leask L, McGee R, et al. 0064 Youth Sleep-Wake Experience in Juvenile Justice Facilities: A Descriptive Analysis. Sleep. 2022;45(Supplement_1):A29–A30. [Google Scholar]
- 56.Gaston KJ, Davies TW, Bennie J, Hopkins J. REVIEW: Reducing the ecological consequences of night-time light pollution: options and developments. Journal of Applied Ecology. 2012;49(6):1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biological Reviews. 2013;88(4):912–27. [DOI] [PubMed] [Google Scholar]
- 58.Aubé M, Roby J, Kocifaj M. Evaluating Potential Spectral Impacts of Various Artificial Lights on Melatonin Suppression, Photosynthesis, and Star Visibility. PLOS ONE. 2013;8(7):e67798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaston KJ, Gaston S, Bennie J, Hopkins J. Benefits and costs of artificial nighttime lighting of the environment. Environmental Reviews. 2015;23(1):14–23. [Google Scholar]
- 60.Rea M, Smith A, Bierman A, Figueiro M. The potential of outdoor lighting for stimulating the human circadian system. [Google Scholar]
- 61.Vollmer C, Michel U, Randler C. Outdoor Light at Night (LAN) Is Correlated With Eveningness in Adolescents. Chronobiology International. 2012;29(4):502–8. [DOI] [PubMed] [Google Scholar]
- 62.Paksarian D, Rudolph KE, Stapp EK, Dunster GP, He J, Mennitt D, et al. Association of Outdoor Artificial Light at Night With Mental Disorders and Sleep Patterns Among US Adolescents. JAMA Psychiatry. 2020;77(12):1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huss A, Wel Lv, Bogaards L, Vrijkotte T, Wolf L, Hoek G, et al. Shedding Some Light in the Dark—A Comparison of Personal Measurements with Satellite-Based Estimates of Exposure to Light at Night among Children in the Netherlands. Environmental Health Perspectives. 2019;127(6):067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607–608:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nadybal SM, Collins TW, Grineski SE. Light pollution inequities in the continental United States: A distributive environmental justice analysis. Environmental Research. 2020;189:109959. [DOI] [PubMed] [Google Scholar]
- 66.Lissak G Adverse physiological and psychological effects of screen time on children and adolescents: Literature review and case study. Environmental Research. 2018;164:149–57. [DOI] [PubMed] [Google Scholar]
- 67.Caumo GH, Spritzer D, Carissimi A, Tonon AC. Exposure to electronic devices and sleep quality in adolescents: a matter of type, duration, and timing. Sleep Health. 2020;6(2):172–8. [DOI] [PubMed] [Google Scholar]
- 68.Bartel K, Gradisar M. New Directions in the Link Between Technology Use and Sleep in Young People. In: Nevšímalová S, Bruni O, editors. Sleep Disorders in Children. Cham: Springer International Publishing; 2017. p. 69–80. [Google Scholar]
- 69.LeBourgeois MK, Hale L, Chang A-M, Akacem LD, Montgomery-Downs HE, Buxton OM. Digital Media and Sleep in Childhood and Adolescence. Pediatrics. 2017;140(Supplement 2):S92–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatori M, Gronfier C, Van Gelder RN, Bernstein PS, Carreras J, Panda S, et al. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. npj Aging and Mechanisms of Disease. 2017;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cajochen C, Dijk D-J, Borbély AA. Dynamics of EEG Slow-Wave Activity and Core Body Temperature in Human Sleep After Exposure to Bright Light. Sleep. 1992;15(4):337–43. [PubMed] [Google Scholar]
- 72.Houser KW, Boyce PR, Zeitzer JM, Herf M. Human-centric lighting: Myth, magic or metaphor? Lighting Research & Technology. 2020;53(2):97–118. [Google Scholar]
- 73.Heath M, Sutherland C, Bartel K, Gradisar M, Williamson P, Lovato N, et al. Does one hour of bright or short-wavelength filtered tablet screenlight have a meaningful effect on adolescents’ pre-bedtime alertness, sleep, and daytime functioning? Chronobiol Int. 2014;31(4):496–505. [DOI] [PubMed] [Google Scholar]
- 74.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44(2):237–40. [DOI] [PubMed] [Google Scholar]
- 75.Figueiro M, Overington D. Self-luminous devices and melatonin suppression in adolescents. Lighting Research & Technology. 2015;48(8):966–75. [Google Scholar]
- 76.Bowler J, Bourke P. Facebook use and sleep quality: Light interacts with socially induced alertness. British Journal of Psychology. 2019;110(3):519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pereira éF, Louzada FM, Moreno CRC. Not all adolescents are sleep deprived: A study of rural populations. Sleep and Biological Rhythms. 2010;8(4):267–73. [Google Scholar]
- 78.Peixoto CAT, da Silva AGT, Carskadon MA, Louzada FM. Adolescents Living in Homes Without Electric Lighting Have Earlier Sleep Times. Behavioral Sleep Medicine. 2009;7(2):73–80. [DOI] [PubMed] [Google Scholar]
- 79.Figueiro MG, Rea MS, Bullough JD. Does architectural lighting contribute to breast cancer? J Carcinog. 2006;5:20-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgess HJ, Molina TA. Home Lighting Before Usual Bedtime Impacts Circadian Timing: A Field Study. Photochemistry and Photobiology. 2014;90(3):723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harada T Effects of evening light conditions on salivary melatonin of Japanese junior high school students. Journal of Circadian Rhythms. 2004;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cain N, Gradisar M, Moseley L. A motivational school-based intervention for adolescent sleep problems. Sleep Medicine. 2011;12(3):246–51. [DOI] [PubMed] [Google Scholar]
- 83.Cain SW, McGlashan EM, Vidafar P, Mustafovska J, Curran SPN, Wang X, et al. Evening home lighting adversely impacts the circadian system and sleep. Scientific Reports. 2020;10(1):19110.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takasu NN, Hashimoto S, Yamanaka Y, Tanahashi Y, Yamazaki A, Honma S, et al. Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1799–807. [DOI] [PubMed] [Google Scholar]
- 85.Wright Kenneth P, McHill Andrew W, Birks Brian R, Griffin Brandon R, Rusterholz T, Chinoy Evan D. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology. 2013;23(16):1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.te Kulve M, Schlangen LJM, van Marken Lichtenbelt WD. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Scientific Reports. 2019;9(1):16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. Journal of pineal research. 2002;33(4):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Estevan I, Tassino B, Vetter C, Silva A. Bidirectional association between light exposure and sleep in adolescents. Journal of Sleep Research.n/a(n/a):e13501. [DOI] [PubMed] [Google Scholar]
- 89.Gasperetti CE, Dolsen MR, Harvey AG. The influence of intensity and timing of daily light exposure on subjective and objective sleep in adolescents with an evening circadian preference. Sleep Medicine. 2021;79:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auger RR, Burgess HJ, Dierkhising RA, Sharma RG, Slocumb NL. Light Exposure Among Adolescents With Delayed Sleep Phase Disorder: A Prospective Cohort Study. Chronobiology International. 2011;28(10):911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson SA, Lack L. Circadian Melatonin and Temperature Taus in Delayed Sleep-wake Phase Disorder and Non-24-hour Sleep-wake Rhythm Disorder Patients: An Ultradian Constant Routine Study. Journal of biological rhythms. 2016;31(4):387–405. [DOI] [PubMed] [Google Scholar]
- 92.Choukroun J, Geoffroy P. Light Therapy in Mood Disorders: A Brief History with Physiological Insights. Chronobiology in Medicine. 2019;1. [Google Scholar]
- 93.Burgess HJ, Emens JS. Circadian-Based Therapies for Circadian Rhythm Sleep-Wake Disorders. Curr Sleep Med Rep. 2016;2(3):158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Misiunaite I, Eastman CI, Crowley SJ. Circadian Phase Advances in Response to Weekend Morning Light in Adolescents With Short Sleep and Late Bedtimes on School Nights. Frontiers in Neuroscience. 2020;14(99). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagare R, Plitnick B, Figueiro MG. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light Res Technol. 2019;51(4):530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18(5):801–8. [DOI] [PubMed] [Google Scholar]
- 97.Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004;36(2):140–4. [DOI] [PubMed] [Google Scholar]
- 98.Figueiro MG, Rea MS. Short-Wavelength Light Enhances Cortisol Awakening Response in Sleep-Restricted Adolescents. International Journal of Endocrinology. 2012;2012:301935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langevin RH, Laurent A, Sauvé Y. Évaluation préliminaire de l’efficacité de la Luminette® chez des adolescents atteints du syndrome de retard de phase du sommeil (SRPS) : essai randomisé en simple insu et contrôlé par placebo. Médecine du Sommeil. 2014;11(2):91–7. [Google Scholar]
- 100.Lovato N, Lack L. Circadian phase delay using the newly developed re-timer portable light device. Sleep and Biological Rhythms. 2016;14(2):157–64. [Google Scholar]
- 101.Kirschbaum-Lesch I, Gest S, Legenbauer T, Holtmann M. Feasibility and Efficacy of Bright Light Therapy in Depressed Adolescent Inpatients. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie. 2018;46(5):423–9. [DOI] [PubMed] [Google Scholar]
- 102.Lau T, Lovato N, Lack L. Evaluation of a portable light device for phase advancing the circadian rhythm in the home environment. Sleep and Biological Rhythms. 2018;16(4):405–11. [Google Scholar]
- 103.Kaplan KA, Mashash M, Williams R, Batchelder H, Starr-Glass L, Zeitzer JM. Effect of Light Flashes vs Sham Therapy During Sleep With Adjunct Cognitive Behavioral Therapy on Sleep Quality Among Adolescents: A Randomized Clinical Trial. JAMA Network Open. 2019;2(9):e1911944-e.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richardson C, Cain N, Bartel K, Micic G, Maddock B, Gradisar M. A randomised controlled trial of bright light therapy and morning activity for adolescents and young adults with Delayed Sleep-Wake Phase Disorder. Sleep Medicine. 2018;45:114–23. [DOI] [PubMed] [Google Scholar]
- 105.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–7. [DOI] [PubMed] [Google Scholar]
- 106.Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, Vedaa O, Nordhus IH, Bjorvatn B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: effects on subjective and objective sleep. Chronobiol Int. 2014;31(1):72–86. [DOI] [PubMed] [Google Scholar]
- 107.Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, Nordhus IH, Vedaa Ø, Lundervold AJ, et al. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. J Biol Rhythms. 2013;28(5):306–21. [DOI] [PubMed] [Google Scholar]
- 108.Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, Menne A, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34(12):1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Danielsson K, Jansson-Fröjmark M, Broman J-E, Markström A. Cognitive Behavioral Therapy as an Adjunct Treatment to Light Therapy for Delayed Sleep Phase Disorder in Young Adults: A Randomized Controlled Feasibility Study. Behavioral Sleep Medicine. 2016;14(2):212–32. [DOI] [PubMed] [Google Scholar]
- 110.Micic G, Richardson C, Cain N, Reynolds C, Bartel K, Maddock B, et al. Readiness to change and commitment as predictors of therapy compliance in adolescents with Delayed Sleep-Wake Phase Disorder. Sleep Med. 2019;55:48–55. [DOI] [PubMed] [Google Scholar]
- 111.Bonnar D, Gradisar M, Moseley L, Coughlin A-M, Cain N, Short MA. Evaluation of novel school-based interventions for adolescent sleep problems: does parental involvement and bright light improve outcomes? Sleep Health. 2015;1(1):66–74. [DOI] [PubMed] [Google Scholar]
- 112.Wada K, Yata S, Akimitsu O, Krejci M, Noji T, Nakade M, et al. A tryptophan-rich breakfast and exposure to light with low color temperature at night improve sleep and salivary melatonin level in Japanese students. Journal of Circadian Rhythms. 2013;11(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wen P, Tan F, Wu M, Cai Q, Xu R, Zhang X, et al. The effects of different bedroom light environments in the evening on adolescents. Building and Environment. 2021;206:108321. [Google Scholar]
- 114.Lawrenson JG, Hull CC, Downie LE. The effect of blue-light blocking spectacle lenses on visual performance, macular health and the sleep-wake cycle: a systematic review of the literature. Ophthalmic Physiol Opt. 2017;37(6):644–54. [DOI] [PubMed] [Google Scholar]
- 115.Esaki Y, Kitajima T, Ito Y, Koike S, Nakao Y, Tsuchiya A, et al. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: An open-label trial. Chronobiol Int. 2016;33(8):1037–44. [DOI] [PubMed] [Google Scholar]
- 116.Perez Algorta G, Van Meter A, Dubicka B, Jones S, Youngstrom E, Lobban F. Blue blocking glasses worn at night in first year higher education students with sleep complaints: a feasibility study. Pilot Feasibility Stud. 2018;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Lely S, Frey S, Garbazza C, Wirz-Justice A, Jenni OG, Steiner R, et al. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J Adolesc Health. 2015;56(1):113–9. [DOI] [PubMed] [Google Scholar]
- 118.Nagare R, Plitnick B, Figueiro M. Does the iPad Night Shift mode reduce melatonin suppression? Lighting Research & Technology. 2019;51(3):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duraccio KM, Zaugg KK, Blackburn RC, Jensen CD. Does iPhone night shift mitigate negative effects of smartphone use on sleep outcomes in emerging adults? Sleep Health. 2021;7(4):478–84. [DOI] [PubMed] [Google Scholar]
- 120.Gentile N, Goven T, Laike T, Sjoberg K. A field study of fluorescent and LED classroom lighting. Lighting Research & Technology. 2018;50(4):631–50. [Google Scholar]
- 121.Keis O, Helbig H, Streb J, Hille K. Influence of blue-enriched classroom lighting on students׳ cognitive performance. Trends in Neuroscience and Education. 2014;3(3):86–92. [Google Scholar]
- 122.Choi K, Suk H-J. The gradual transition from blue-enriched to neutral white light for creating a supportive learning environment. Building and Environment. 2020;180:107046. [Google Scholar]
- 123.Studer P, Brucker JM, Haag C, Van Doren J, Moll GH, Heinrich H, et al. Effects of blue- and red-enriched light on attention and sleep in typically developing adolescents. Physiology & Behavior. 2019;199:11–9. [DOI] [PubMed] [Google Scholar]
- 124.Jarboe C Research: Strategies for delivering circadian stimulus in a classroom while minimizing energy use. LD+A Online [Internet]. 2021. Available from: https://www.ies.org/lda/research-strategies-for-delivering-circadian-stimulus-in-a-classroom-while-minimizing-energy-use/. [Google Scholar]
- 125.Stefani O, Freyburger M, Veitz S, Basishvili T, Meyer M, Weibel J, et al. Changing color and intensity of LED lighting across the day impacts on circadian melatonin rhythms and sleep in healthy men. Journal of Pineal Research. 2021;70(3):e12714. [DOI] [PubMed] [Google Scholar]
- 126.Papatsimpa C, Bonarius JH, Linnartz JPMG, editors. Human Centric IoT Lighting Control based on Personalized Biological Clock Estimations. 2020 IEEE 6th World Forum on Internet of Things (WF-IoT); 2020 2–16 June 2020. [Google Scholar]
- 127.Schratz M, Gupta C, Struhs TJ, Gray K. A New Way to See the Light: Improving Light Quality with Cost-Effective LED Technology. IEEE Industry Applications Magazine. 2016;22(4):55–62. [Google Scholar]
- 128.Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proceedings of the National Academy of Sciences. 2019;116(24):12019. [DOI] [PMC free article] [PubMed] [Google Scholar]