Abstract
Tigilanol tiglate is a natural product diterpenoid in clinical trials for the treatment of a broad range of cancers. Its unprecedented protein kinase C isoform selectivity make it and its analogues exceptional leads for PKC-related clinical indications, which include human immunodeficiency virus and AIDS eradication, antigen-enhanced cancer immunotherapy, Alzheimer’s disease and multiple sclerosis. Currently, the only source of tigilanol tiglate is a rain forest tree, Fontainea picrosperma, whose limited number and restricted distribution (northeastern Australia) has prompted consideration of designed tree plantations to address supply needs. Here we report a practical laboratory synthesis of tigilanol tiglate that proceeds in 12 steps (12% overall yield, >80% average yield per step) and can be used to sustainably supply tigilanol tiglate and its analogues, the latter otherwise inaccessible from the natural source. The success of this synthesis is based on a unique strategy for the installation of an oxidation pattern common to many biologically active tiglianes, daphnanes and their analogues.
Ligands that modulate protein kinase C (PKC) signalling1 have been implicated in therapeutic approaches to human immunodeficiency virus and AIDS eradication2, antigen-enhanced antibody and chimeric antigen receptor (CAR) T-cell therapies3,4, suppression of T-cell exhaustion in cancer immunotherapy5, Alzheimer’s disease6 and multiple sclerosis7. Some modulators have advanced towards clinical evaluation8,9, such as tigilanol tiglate (1, EBC-46), a naturally occurring tigliane diterpenoid recently evaluated in phase I clinical trials for the treatment of a broad range of cancers in humans and currently in trials for head and neck squamous cell carcinomas10. Intratumoural injection of EBC-46 induces rapid tumour ablation, in part by a proposed isoform-selective modulation of PKC11,12. After administration, EBC-46 induces a localized immune response and rupture of tumour vasculature, which leads to haemorrhagic necrosis, subsequent clearance of the solid tumour and facilitated wound healing13,14. Recently, EBC-46, branded Stelfonta, received approval by the US Food and Drug Administration15 for the treatment of non-metastatic mast cell tumours in canines. In a recent clinical study, a 75% complete response was observed in canines after a single intratumoural injection and 88% remission after a second dose16, which prompted its current evaluation in human trials.
Currently, the only source of EBC-46 is the dioecious blushwood tree (Fontainea picrosperma), a rainforest Euphorbiaceae, limited in number and endemic to a small region of northeastern Australia17,18. As reported, to access EBC-46 and ester variants from rain forest tree seeds, the seeds are extracted with ethanol and the resultant extract is partitioned between petroleum ether and water. The contents of the organic phase are then converted into EBC-46 using six chromatographic purifications and five low-yielding synthetic steps (~5% yield)19. Prompted by its limited natural source, environmental considerations and its emerging clinical value, efforts to improve EBC-46 production have been directed at cultivating its source plant, F. picrosperma, in designed plantations17. However, this source, although it avoids rain forest harvesting, is still pollinator limited and at risk of disruption by climate variations and invasive pathogens18,20. More geographically distributed and diverse sources would offer a more sustainable supply for research and clinical needs.
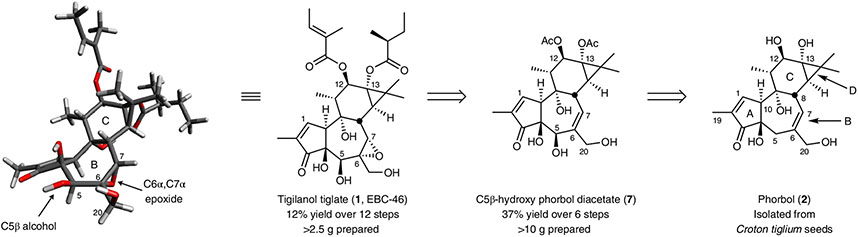
Given the immediate clinical and research value of EBC-46 and its analogues, a practical and more sustainable solution to the supply problem could be realized through a time- and step-economical21 semisynthesis from a more available and diversified source22. Similar strategies that combine the power of biological and chemical synthesis enable rapid access to other clinical candidates, such as Taxol and prostratin and their analogues22-26. Towards this end, phorbol esters represent potential precursors to EBC-46. Although available through total synthesis27-29, they are even more readily accessed from more than 7,000 species of the globally distributed Euphorbiaceae and Thymelaeceae plant families30. Although plant cultivars vary in phorbol ester content, the seeds of the Croton tiglium plant of the Euphorbiaceae family supply upwards of 1.6% w/w of phorbol (2) upon extraction and ester hydrolysis31. Given the low cost (~US$40 kg−1) of these seeds and the diverse geographical distribution of their varied sources, we set out to design a synthetic route to EBC-46 based on phorbol (2) as the starting material (Fig. 1). To obtain this material efficiently, we developed an improved scalable isolation protocol building on prior work32,33 that, on average, afforded >10 g of phorbol (2) from 3 kg of seeds (Supplementary page SI-5). This isolation protocol consists of grinding the seeds and base-mediated removal of the C20, C12 and C13 esters in the extract to produce an oil from which phorbol (2) is purified by column chromatography.
Fig. 1 ∣. Structural analysis of tigilanol tiglate (1) and a retrosynthetic analysis of its synthesis from phorbol (2).
Over 10 g of diversifiable intermediate 7 was prepared from phorbol (2), which was isolated in decagram quantities from C. tiglium seeds. The three-dimensional structure of 1 was calculated using Macromodel (Schrödinger Suite 2016) and optimized with density functional theory (M06-2X3).
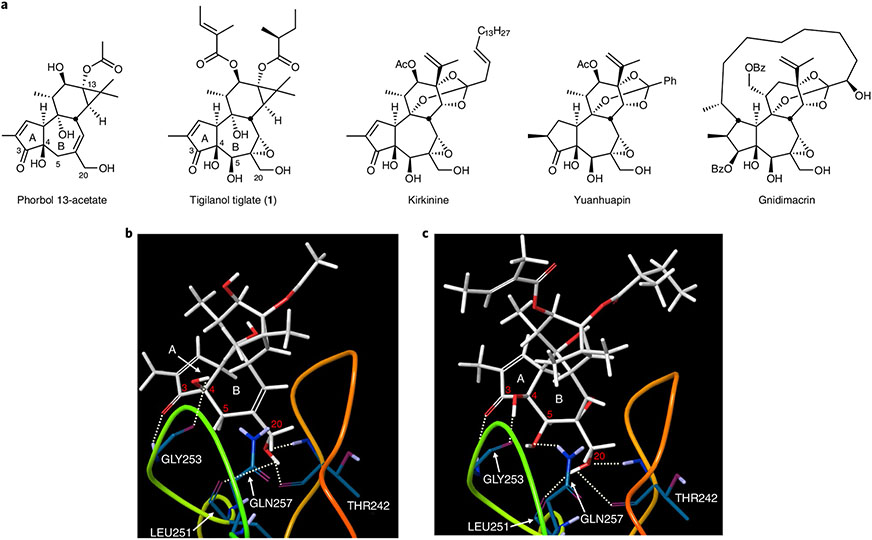
A key challenge associated with synthetically accessing EBC-46 and many related, biologically active tigliane and daphnane natural products is the construction of their common B-ring 5β-hydroxy-6α,7α-epoxy functionality (Fig. 2a)30,34. Based on our original pharmacophore model, we expect that this functionality, among other B-ring functional groups, influences PKC affinity, selectivity and function (Fig. 2b)35,36. Thus, the core of this problem is oxy-functionalization of the β-5 allylic hydrogen in the presence of other allylic hydrogens at C8, C20, C10 and C19. This problem is further exacerbated by phorbol’s sensitivity to heat, light, acid, base and air oxidation37. Attempts at direct CH activation at C5 have thus far failed33,38. With a scalable source of phorbol (2) in hand, we now describe a solution to this problem that provides, in six steps, scalable access to a highly diversifiable intermediate 7 (Fig. 3) from which EBC-46 and new analogues are readily derived.
Fig. 2 ∣. Overview of the importance of the B-ring oxidation pattern in tigliane and daphnane natural products and the pharmacophore model.
a, The structures of phorbol 13-acetate and of representative members of the tigliane and daphnane families with a shared B-ring functionality. b, X-ray crystal structure of phorbol 13-acetate bound to the C1 domain of PKC-δ35,36. c, Predicted binding mode of EBC-46 to the C1 domain of PKC-δ. Dotted lines represent hydrogen bonds.
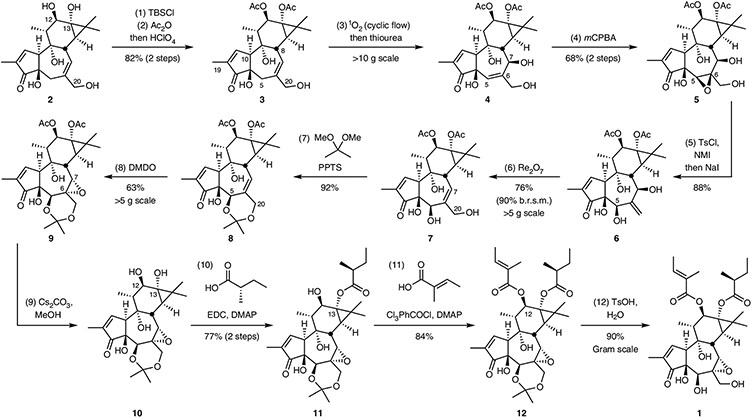
Fig. 3 ∣. Reaction sequence from phorbol (2) to tigilanol tiglate (1).
Reagents and conditions. (1) C20 silylation: tert-butyldimethylsilyl chloride (TBSCl) (7 equiv.), imidazole (15 equiv.), dimethylformamide, 0 °C. (2) C12,C13 acetylation and C20 desilylation: acetic anhydride (Ac2O) (15 equiv.), triethylamine (NEt3) (15 equiv.), 4-dimethylaminopyridine (0.3 equiv.), CH2Cl2; then MeOH, 0 °C to room temperature; then HClO4 (25 equiv.). (3) C7 singlet oxygen ene reaction (cyclic flow, approximately 100 cycles of 5 min, reaction progress tracked by thin-layer chromatography and/or NMR spectroscopy; for more information, see Supplementary pages SI-13 and SI-14.); Rose bengal (1.5 mM), O2, CD3OD, 20 °C; then thiourea (3 equiv.). (4) C5,C6 epoxidation: mCPBA (2 equiv.), 3:1 CH2Cl2:ether, 4 °C. (5) C20 tosylation and reductive epoxide opening: p-toluenesulfonyl chloride (TsCl) (1.2 equiv.), NMI (0.1 equiv.), NEt3 (1.5 equiv.), acetonitrile, 0 °C; then H2O; then sodium iodide (NaI) (3 equiv.), 60 °C. (6) C7,C20 allylic transposition: rhenium(VII) oxide (Re2O7), (0.10 equiv.), THF, 4 °C. (7) C5,C20 acetonide protection: 2,2-dimethoxypropane (300 equiv.), pyridinium p-toluenesulfonate (PPTS) (0.15 equiv.), acetone; then rotovap; then acetone (8) C6,C7 epoxidation: dimethyldioxirane (DMDO) (3 equiv.), acetone. (9) C12,C13 deacetylation: Cs2CO3 in methanol (pH = 11). (10) C13 esterification: (S)-2-methylbutanoic acid (3 equiv.), 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDC) (3.15 equiv.), NEt3 (3.30 equiv.), 4-dimethylaminopyridine (DMAP) (0.2 equiv.), CH2Cl2. (11) C12 esterification: tiglic acid (2.2 equiv.), 2,4,6-trichlorobenzoyl chloride (Yamaguchi reagent) (2 equiv.), NEt3 (4 equiv.), DMAP (2.6 equiv.), toluene. (12) C5,C20 acetonide deprotection: p-toluenesulfonic acid in water (1 M), acetonitrile. b.r.s.m., based on recovered starting material.
Results and discussion
Anticipating that esters at C12 and C13 could be exchanged by late-stage diversification and would minimize interference with B-ring modifications, we opted to start with the simple diacetate 3, which is prepared from phorbol (2) with an 82% yield via t-butyldimethylsilyl protection at C20 followed by acetylation at C12 and C13 and a desilylative workup (Fig. 3). Efforts to convert this two-step process into one step via triacetylation and selective deprotection of the C20 acetate gave lower yields (~65%) and was not utilized on large scale.
Chemo-, regio- and stereoselective oxidation at C5 of diacetate 3 in the presence of potentially oxidizable allylic sites at C8, C10, C19 and C20 was efficiently realized using a photosensitized singlet oxygen ene reaction with Rose bengal as the photosensitizer, green light-emitting diodes (λ = 535 nm) as the photon source39,40 and methanol-d4 as the solvent, which minimizes singlet oxygen destruction40. In situ reduction of the resultant hydroperoxide initially produced allylic alcohol 4 in moderate yield (66%). Although this reaction can be routinely performed batch-wise on small scales (<500 mg), large-scale batch reactions suffered from light penetration issues and raised concerns about the accumulation of the potentially unstable hydroperoxide intermediate39. To address these scalability problems, we assembled a cyclic flow photoreactor that utilized a peristaltic pump and Tygon tubing (Supplementary Figs. 3 and 4)41. Using this apparatus, we produced the ene product 4 on a decagram scale (for example, 19 g) in an 88% yield as determined by quantitative NMR. Although further purified for characterization purposes, this compound was sufficiently pure to be used directly in the following step thereby avoiding chromatographic purification.
It was envisioned that 4 could be converted into the C5 alcohols 6 or 7 via rhenium-catalysed allylic transposition42,43. However, the reaction of 4 using literature conditions was sluggish and provided only minor amounts of the undesired C5α-hydroxy-C6,C20 alkene. As an effective alternative route to 6, we found that epoxidation of the C5,C6 alkene in 4 with m-chloroperbenzoic acid (mCPBA) proceeded preferentially from the sterically more accessible β-face to give epoxide 5, with the desired C5β-O bond, in 77% yield. N-methylimidazole (NMI)-catalysed chemoselective tosylation of the primary C20 alcohol and subsequent reaction with sodium iodide gave exclusively the desired β-C5 alcohol 6 in 88% yield44.
On treatment with catalytic Re2O7, the bis-allylic alcohol 6 underwent a highly chemoselective 1,3-allylic alcohol transposition42,43 to afford C5β-hydroxy phorbol diacetate 7 in a 76% yield (90% based on recovered 6), which serves as a diversification node21,24 to access unexplored B-ring analogues of 1. Other rhenium catalysts led to complex mixtures or a lower conversion (Supplementary Table 1).
Subsequent epoxidation of the C6,C7 alkene of 7 occurred only on the sterically more accessible (undesired) β-face, as expected from our previous work45,46. Although chiral catalyst-controlled epoxidation might address this selectivity problem46, we found a more effective solution; specifically, the facial selectivity exhibited by 7 can be dramatically reversed by the conversion of 7 into its acetonide 8 (92%). Models suggest that the acetonide between the C5 and C20 alcohols induces a conformational change in the B-ring that makes the β-face more sterically encumbered and the α-face less so. Additionally, this protection of the C5 and C20 alcohols serves to simplify subsequent functionalization of the C12 and C13 alcohols. Although the initial epoxidation of acetonide 8 with mCPBA under a variety of conditions (Supplementary Table 2) was slow and low yielding, we found that treatment with the sterically smaller and more reactive dimethyldioxirane (DMDO) stereoselectively gave α-epoxide 9 in a 63% yield. This substrate-controlled facially selective epoxidation is unprecedented for this class of compounds and thus provides a potentially general method to access other structurally similar and biologically active tigliane and daphnane diterpenoids30,34.
Deacetylation of diester 9 provided the corresponding C12,C13 diol 10 in an 86% yield as determined by quantitative NMR. Although further purified for characterization purposes, this compound was sufficiently pure to be used directly in the following step thereby avoiding chromatographic purification. Diol 10 serves as a second point of diversification for C12,C13 derivatization, now with the desired C5β-hydroxy-C6α,C7α-epoxy B-ring in place11,24,36. From 10, EBC-46 was prepared on gram scale via selective diesterification47 and acidic deprotection of the acetonide. In our laboratory, this overall route and greatly improved final esterification sequence delivered over 2.5 g of EBC-46 (Supplementary Fig. 7). All the steps were performed by two or more investigators to ensure reproducibility. Collectively, our synthetic strategy provides access to B-ring analogues from intermediate 7, A-ring analogues from intermediates 7–12 and C-ring analogues from intermediate 10.
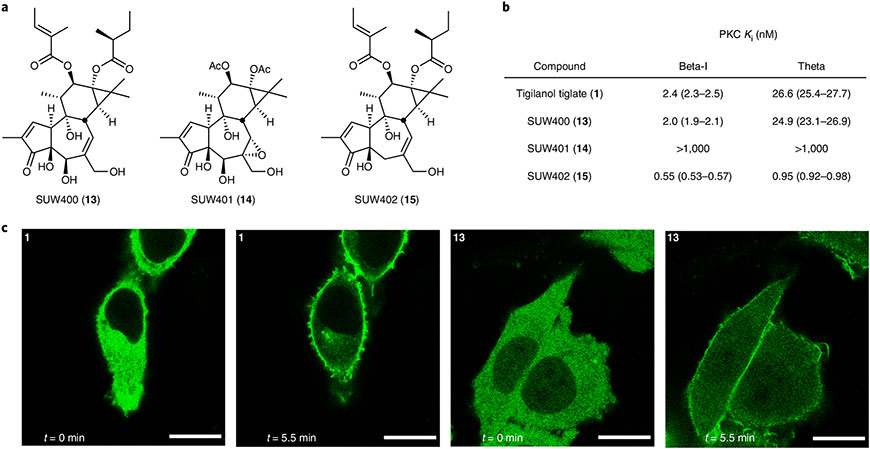
Although a comprehensive analysis of the binding, selectivity and biological activities of various analogues will be disclosed separately, it is noteworthy that even modest structural changes dramatically affect PKC affinity and selectivity. To begin our investigation into the role of the C5β-hydroxy-C6α,C7α-epoxy functionality and the C12,C13 esters in determining PKC affinity and selectivity, we prepared an initial series of analogues (Fig. 4a). These analogues, along with EBC-46, were tested for their affinity to PKC-βI and PKC-θ, representative conventional and novel isoforms of PKC, respectively (Fig. 4b). Specifically, to determine the role of the C6α,C7α-epoxide in PKC binding and selectivity, we synthesized a C6,C7-alkene analogue (13, SUW400), otherwise inaccessible from EBC-46. Interestingly, this analogue exhibited a nearly identical binding affinity and selectivity to PKC-βI and PKC-θ when compared with that of EBC-46. This finding suggests that the C6α,C7α-epoxide is not necessary for the isoform-selective binding exhibited by EBC-46. Similarly, to determine the role of the C5β-alcohol in the PKC binding and selectivity, we synthesized a C5-deoxy-C6,C7-alkene analogue (15, SUW402). This analogue showed a stronger but less selective binding than that of both EBC-46 and SUW400. This finding suggests that the C5β-alcohol plays an important role in isoform binding selectivity. Finally, to begin investigating the role of the C12,C13 esters in PKC binding and selectivity, we synthesized a diacetate analogue (14, SUW401). This analogue showed a very low PKC binding affinity when compared with that of EBC-46. This finding suggests that the C12,C13 esters also play an important role in PKC binding. Given the potent PKC affinity of EBC-46, SUW400 and SUW402, these compounds were tested in vitro for their ability to permeate CHO-K1 (Chinese hamster ovary factor K1) cells and translocate, in real time, an optically tagged PKC fusion protein (PKC-GFP; GFP, green fluorescent protein) from the cytosol to the membrane–the hallmark of PKC activation1 (Supplementary Fig. 8). The details and experimental procedures for this assay were published previously48. EBC-46 showed a modest translocation of PKC-βI-GFP at low (200 nM) concentrations and a robust translocation at high (1,000 nM) ones (Fig. 4c). Interestingly, the more synthetically accessible SUW400 and SUW402 showed a comparable translocation to that of EBC-46 at low (200 nM) as well as high (1,000 nM) concentrations. Future studies on these and other analogues, readily accessible from our synthetic route, are directed at the elucidation of the structural basis for isoform-selective PKC modulation and the role of isoform selectivity in human immunodeficiency virus and AIDS latency reversal, tumour ablation, antigen enhancement for antigen-targeted antibody and chimeric antigen receptor cell therapies, suppression of T-cell exhaustion and neurological disorders.
Fig. 4 ∣. Representative biological data for synthetic EBC-46 and its analogues.
a, Structures of EBC-46 analogues. b, Cell-free PKC binding data for 1, 13, 14 and 15. The unique PKCβ-selective binding mode of 1 is mimicked by 13, whereas 14 exhibits no meaningful PKC binding and 15 binds in a potent and unselective fashion. c, In-vitro PKC-βI-GFP translocation in CHO-K1 cells mediated by 1 and 13 (1,000 nM). Scale bars, 10 μm). Upon exposure to PKC modulators 1 and 13 for 5 min, the GFP-labelled PKC was observed to translocate from the middle of the cells (cytosol) to the periphery (cell membrane).
In summary, we describe a scalable laboratory preparation of tigilanol tiglate (1, EBC-46), an approved veterinary therapeutic and a human clinical lead for cancer and other indications. Previously, tigilanol tiglate was considered synthetically inaccessible and only available from a limited natural source, the latter raising environmental concerns. Our synthetic strategy also enables access to numerous biologically active tiglianes, daphnanes and their analogues. This strategy will accelerate future studies directed at the structural basis for PKC isoform selectivity and its role in mode of action and disease-specific activities.
Methods
As the hazard of new compounds is unknown, all the procedures were conducted with full personal protective equipment in a way that avoids exposure. CHO-K1 (ATCC) was the cell line used for translocation experiments. No commonly misidentified cell lines were used in this study. None of the cell lines used were authenticated. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications4.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH) (CA31845 and AI124743; Z.O.G., D.J.F., O.D.M., Q.H.L.-N and E.N.). O.D.M. thanks the Molecular Pharmacology Training Program for support. We also thank H. Rahn for thoughtful discussions and assistance in the purification. Confocal images were acquired at the Stanford Neuroscience Microscopy Services. High-resolution mass spectrometric data were acquired at the Vincent Coates Foundation Mass Spectrometry Laboratory, supported in part by NIH P30 CA124435 utilizing the Stanford Cancer Institute Proteomics/Mass Spectrometry Shared Resource. Computational efforts were performed on the Sherlock cluster (Stanford University).
Footnotes
Competing interests
A provisional patent application (docket number S21-064) has been filed by Stanford University, on behalf of Paul A. Wender (principal investigator), Zachary O. Gentry, David J. Fanelli, Quang H. Luu-Nguyen, Owen D. McAteer and Edward Njoo, that covers a method to synthesize tigilanol tiglate (EBC-46) and related compounds from readily available starting materials.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41557-022-01048-2.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41557-022-01048-2.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. The X-ray structure of phorbol-13-acetate bound to the PKC-C1 domain was obtained from the structure reported by Hurley (Protein Data Bank: 1PTR).
References
- 1.Newton AC & Brognard J Reversing the paradigm: protein kinase C as a tumor suppressor. Trends Pharmacol. Sci 38, 438–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JT et al. Latency reversal plus natural killer cells diminish HIV reservoir in vivo. Nat. Commun 13, 121 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishna S et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin. Cancer Res 25, 5329–5341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardman C et al. Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy. Nat. Commun 11, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marro BS et al. Discovery of small molecules for the reversal of T-cell exhaustion. Cell Rep. 29, 3293–3302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M-K, Hongpaisan J, Lim CS & Alkon DL Bryostatin-1 restores hippocampal synapses and spatial learning and memory in adult fragile X mice. J. Pharmacol. Exp. Ther 349, 393–401 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Kornberg MD et al. Bryostatin-1 alleviates experimental multiple sclerosis. Proc. Natl Acad. Sci. USA 115, 2186–2191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez C et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30, 1385–1392 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Farlow MR et al. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J. Alzheimers Dis 67, 555–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panizza BJ et al. Phase I dose-escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC-46). EBioMedicine 50, 433–441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen JK et al. Activation of PKC supports the anticancer activity of tigilanol tiglate and related epoxytiglianes. Sci Rep. 11, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller J et al. Dose characterization of the investigational anticancer drug tigilanol tiglate (EBC-46) in the local treatment of canine mast cell tumors. Front. Vet. Sci 6, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses RL et al. Novel epoxy-tiglianes stimulate skin keratinocyte wound healing responses and re-epithelialization via protein kinase C activation. Biochem. Pharmacol 178, 114048 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Boyle GM et al. Intra-lesional injection of the novel PKC activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE 9, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA Approves First Intratumoral Injection to Treat Non-Metastatic Mast Cell Tumors in Dogs https://www.fda.gov/news-events/press-announcements/fda-approves-first-intratumoral-injection-treat-non-metastatic-mast-cell-tumors-dogs (2020).
- 16.De Ridder TR et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC-46). J. Vet. Intern. Med 35, 415–429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamont RW, Conroy GC, Reddell P & Ogbourne SM Population genetic analysis of a medicinally significant Australian rainforest tree, Fontainea Picrosperma C.T. White (Euphorbiaceae): biogeographic patterns and implications for species domestication and plantation establishment. BMC Plant Biol. 16, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant EL et al. Floral attraction and flower visitors of a subcanopy, tropical rainforest tree, Fontainea Picrosperma. Ecol. Evol 11, 10468–10482 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul I, Reddell W, Gordon VA Tiglien-3-one derivatives. US Patent 9770431B2 (2017).
- 20.Grant E Reproductive Biology, Flowering and Genetics of Fontainea picrosperma (Euphorbiaceae). PhD thesis, Univ. Sunshine Coast; (2020). [Google Scholar]
- 21.Wender PA, Quiroz RV & Stevens MC Function through synthesis-informed design. Acc. Chem. Res 48, 752–760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z & Hui C Contemporary advancements in the semi-synthesis of bioactive terpenoids and steroids. Org. Biomol. Chem 19, 3791–3812 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Wender PA, Verma VA, Paxton TJ & Pillow TH Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res 41, 40–49 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Kim KE, Kim AN, McCormick CJ & Stoltz BM Late-stage diversification: a motivating force in organic synthesis. J. Am. Chem. Soc 143, 16890–16901 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman DJ & Cragg GM Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod 83, 770–803 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Liu WC, Gong T & Zhu P Advances in exploring alternative Taxol sources. RSC Adv. 6, 48800–48809 (2016). [Google Scholar]
- 27.Wender PA, Rice KD & Schnute ME The first formal asymmetric synthesis of phorbol. J. Am. Chem. Soc 119, 7897–7898 (1997). [Google Scholar]
- 28.Lee K & Cha JK Formal synthesis of (+)-phorbol. J. Am. Chem. Soc 123, 5590–5591 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Kawamura S, Chu H, Felding J & Baran PS Nineteen-step total synthesis of (+)-phorbol. Nature 532, 90–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HB, Wang XY, Liu LP, Qin GW & Kang TG Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem. Rev 115, 2975–3011 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Ahmed WA & Salimon J Phorbol ester as toxic constituents of tropical Jatropha curcas seed oil. Eur. J. Sci. Res 31, 429–436 (2009). [Google Scholar]
- 32.Pagani A, Gaeta S, Savchenko AI, Williams CM & Appendino G An improved preparation of phorbol from croton oil. Beilstein J. Org. Chem 13, 1361–1367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann T, Franzyk H & Christensen SB Phorbol rearrangements. J. Nat. Prod 81, 2134–2137 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Hou Z, Yao G & Song S Daphnane-type diterpenes from genus Daphne and their anti-tumor activity. Chin. Herbal Medicines 13, 145–156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Kazanietz MG, Blumberg PM & Hurley JH Crystal structure of the Cys2 activator-binding domain of protein kinase Cδ in complex with phorbol ester. Cell 81, 917–924 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Wender PA, Donneley AC, Loy BA, Near KE, Staveness D in Natural Products in Medicinal Chemistry (ed. Hanessian S) 475–544 (Wiley-VCH, 2014). [Google Scholar]
- 37.Schmidt R & Hecker E Autoxidation of phorbol esters under normal storage conditions. Cancer Res. 35, 1375–1377 (1994). [PubMed] [Google Scholar]
- 38.Amin HIM et al. The allylic oxidation of tigliane esters. Fitoterapia 148, 104802 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Ghogare AA & Greer A Using singlet oxygen to synthesize natural products and drugs. Chem. Rev 116, 9994–10034 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Sagadevan A, Hwang KC & Su M-D Singlet oxygen-mediated selective C─H bond hydroperoxidation of ethereal hydrocarbons. Nat. Commun 8, 1812 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lévesque F & Seeberger PH Highly efficient continuous flow reactions using singlet oxygen as a ‘green’ reagent. Org. Lett 13, 5008–5011 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Volchkov I & Lee D Recent developments of direct rhenium-catalyzed [1,3]-transpositions of allylic alcohols and their silyl ethers. Chem. Soc. Rev 43, 4384–4394 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Morrill C, Beutner GL & Grubbs RH Rhenium-catalyzed 1,3-isomerization of allylic alcohols: scope and chirality transfer. J. Org. Chem 71, 7813–7825 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Ferrier RJ & Hall DW One-step synthesis of glycosidic spiroketals from 2,3-epoxybutyl glycoside derivatives. J. Chem. Soc. Perkin Trans 1992, 3029–3034 (1992). [Google Scholar]
- 45.Wender PA et al. Gateway synthesis of daphnane congeners and their protein kinase C affinities and cell-growth activities. Nat. Chem 3, 615–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boudreault PL, Mattler JK & Wender PA Studies on the regio- and diastereo-selective epoxidation of daphnanes and tiglianes. Tetrahedron Lett. 56, 3423–3427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson TC et al. Synthesis of Eupalinilide E, a promoter of human hematopoietic stem and progenitor cell expansion. J. Am. Chem. Soc 138, 6068–6073 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Benner NL et al. Functional DNA delivery enabled by lipid-modified charge-altering releasable transporters (CARTs). Biomacromolecules 19, 2812–2824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information. The X-ray structure of phorbol-13-acetate bound to the PKC-C1 domain was obtained from the structure reported by Hurley (Protein Data Bank: 1PTR).