Eegenskapen
Algemian
Nööm, Symbool, Numer
Swaawel, S, 16
Seerie
Nianmetal
Skööl, Periode, Blook
16 , 3 , p
Klöör, Skak
güül
CAS-Numer
7704-34-9
ATC-Code
D10 AB02
Uundial
0,048 %[ 1]
Atomaar [ 2]
Atoommase
32,06 (32,059 – 32,076)[ 3] [ 4] u
Atoomraadius (bereegent)
100 (88) pm
Kovalent-Raadius
102,5 pm
Van der Waals-Raadius
180 pm
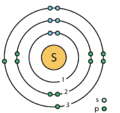
Elektroonen
[Ne ] 3s 2 3p 4
1. Ionisiarang
10. eV [ 5] ≈ . kJ /mol [ 6] kJ /mol
2. Ionisiarang
. [ 5] 2 251. [ 6]
3. Ionisiarang
. [ 5] 3 363. [ 6]
4. Ionisiarang
. [ 5] 4 556. [ 6]
5. Ionisiarang
. [ 5] 7 004. [ 6]
6. Ionisiarang
. [ 5] 8 495. [ 6]
Füsikaalisk [ 2]
Tustant
fest
Kristal
orthorhombisk
Sachthaid
2,07 g/cm³[ 7]
Hardhaid
2
Magnetismus
diamagneetisk (Χm −5 )[ 8]
Smoltponkt
388,36 K (115,21 °C)
Köögponkt
718,2 K[ 9] K (445 °C)
Molaar Rüm
15,53 · 10−6 m3 /mol
Dampwaremk
45 kJ/mol[ 9]
Smoltwaremk
1,713 kJ/mol
Waremk
736[ 1]
Waremkfeerang
0,205 W /(m · K)
Cheemisk [ 2]
Oksidatsionstustant
−2 bis +6
Normoolpotentiaal
−0,48 V (S + 2 e− → S2− )
Elektronegatiwiteet
2,58 (Pauling-Skala)
Isotoopen
Isotoop
NH
t1/2
Aktiwiteet
Energii (MeV )
Produkt
30 S
{syn.}
1,178 s ε 6,138 30 P
31 S
{syn.}
2,572 s ε 5,396 31 P
32 S
95,02 %
stabiil
33 S
0,75 %
stabiil
34 S
4,21 %
stabiil
35 S
{syn.}
87,32 d β− 0,167 35 Cl
36 S
0,02 %
stabiil
37 S
{syn.}
5,05 min β− 4,865 37 Cl
38 S
{syn.}
170,3 min β− 2,937 38 Cl
39 S
{syn.}
11,5 s β− 6,640 39 Cl
40 S
{syn.}
8,8 s β− 4,710 40 Cl
Muar isotoopen bi List faan isotoopen
NMR -Eegenskapen
Seekerhaid
Miast wurd SI -ianhaiden brükt.
Swaawel as en cheemisk element mä det ufkörtang S an det atoomnumer 16 . Hat hiart tu a nianmetalen .
SIMS -Spektrum faan a
isotoopen .
Swaawel
Elektroonenskel
Swaawelstooker
↑ 1,0 1,1 Harry H. Binder: Lexikon der chemischen Elemente. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
↑ Wäärser kem faan www.webelements.com (Schwefel) .
↑ CIAAW, Standard Atomic Weights Revised 2013 .↑ IUPAC, Standard Atomic Weights Revised 2013 (Excel-Tabelle) .↑ 5,0 5,1 5,2 5,3 5,4 5,5 Eintrag zu sulfur NIST Atomic Spectra Database (ver. 5.7.1) . Hrsg.: NIST , Gaithersburg, MD. doi :10.18434/T4W30F https://physics.nist.gov/asd ). Afrepen di 11. Jüüne 2020.
↑ 6,0 6,1 6,2 6,3 6,4 6,5 Eintrag zu sulfur WebElements, https://www.webelements.com , ufrepen di 11. Jüüne 2020.
↑ S. J. Rettig, J. Trotter: Refinement of the Structure of Orthorhombic Sulfur, α-S8 . Uun: Acta Crystallographica Section C. Band 43, 1987, S. 2260–2262, doi:10.1107/S0108270187088152 .
↑ (ütjden faan) David R. Lide: CRC Handbook of Chemistry and Physics .Properties of the Elements and Inorganic Compounds, S. 4-142 – 4-147.
↑ 9,0 9,1 Yiming Zhang, Julian R. G. Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. Uun: Journal of Chemical & Engineering Data. 56, 2011, S. 328–337, doi:10.1021/je1011086 .
↑ Iindraanj tu Schwefel uun't GESTIS-dootenbeenk faan't IFA , ufrepen di 9. August 2016 (mä JavaScript) .↑ Iindrach tu Sulfur Classification and Labelling Inventory faan't Europeesk Chemikaalienagentuur (ECHA), ufrepen di 1. August 2016.