Practice Essentials
In this article, pediatric mucormycosis will be reviewed, such as infection involving immunosuppressed children; this disease has also been observed in neonates (especially premature infants), patients with burns, and children with a history of incidental trauma.
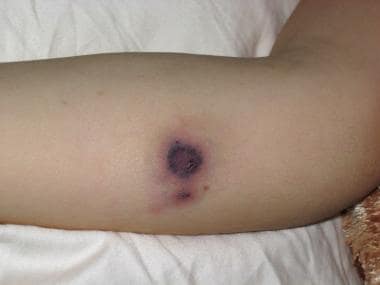
Mucormycosis usually refers to fungal infections in immunosuppressed hosts caused by ubiquitous molds found in organic matter and soil. Such molds belong to the order Mucorales. [1, 2, 3] The infections they cause manifest in the rhinocerebral, pulmonary, cutaneous (see the image below), gastrointestinal (GI), disseminated, and central nervous systems. [4]
Mucormycosis is often life threatening. Therefore, prompt diagnosis and institution of antifungal therapy are vital, as is appropriate management of the underlying disease process.
See also Mucormycosis (Zygomycosis) and Rhinocerebral Mucormycosis.
Pathophysiology
The fungus of mucormycosis gains entry into the body through the nasopharynx. It can be inhaled into the lungs, or it can extend to the sinuses, orbit, and brain. The occurrence of mucormycosis depends on host immunity, but the mechanisms of increased susceptibility in certain hosts remain perplexing. Regardless of the anatomic site involved, characteristic histopathologic findings include angioinvasion with subsequent tissue infarction and necrosis leading to tissue destruction. [5]
The fungal pathogens that cause mucormycosis belong to the class Zygomycetes and the order Mucorales. Rhizopus species are the agents most commonly isolated in mucormycosis, followed by Rhizomucor species, Absidia corymbifera, Apophysomyces elegans, Cunninghamella bertholletiae, Mucor species, and Saksenaea vasiformis. [1, 2, 3]
Etiology
Immunodeficiency
Risk factors for mucormycosis in adults and children include diabetes mellitus (especially with ketoacidosis), which is the underlying condition most commonly associated with this disease. This feature is probably due to diminished function of phagocytes at low pH.
Other individuals at risk include patients with malignancy, those with protein-calorie malnutrition, those with skin breakdown due to burns, those with trauma or who are undergoing surgery, those with acute and chronic renal disease, and those with hematologic disease who are receiving deferoxamine.
Patients with immunosuppression due to acquired immunodeficiency syndrome (AIDS), organ transplantation, neutropenia, or steroid therapy are also at risk.
Nosocomial infection
Hospital-acquired mucormycosis has been reported. In a review of 26 hospitalized, posttraumatic patients with mucormycosis, approximately 57% were females and two thirds had comorbidities (ie, diabetes mellitus, leukemia, immunosuppression); diabetes was noted in 6 patients. [6] In hospitalized patients with cannula, wound or occlusive dressings should be closely watched for erythema or necrosis.
COVID-19
The incidence of COVID-19–associated mucormycosis has increased in India, with more than 20,000 cases reported. [7] A retrospective study conducted in India from September-December 2020 found a 2.0-fold rise in 2020 (compared with 2019) of COVID-19 mucormycosis, with a prevalence of 0.27% among hospitalized patients with COVID-19. The most common clinical manifestation was the rhino-orbital form. [8]
Neonatal infection and prematurity
Neonates, especially those born prematurely, can be at risk. Unusual incidents of neonatal mucormycosis might have occurred when patients were exposed to contaminated surfaces, such as bandages, tongue depressors used as arm splints, or cardiac-monitor leads. [9]
Epidemiology
In the United States, mucormycosis is most common in immunocompromised hosts, although cases in immunocompetent patients are also reported. Underlying diseases, such as diabetes mellitus and malignancy, are risk factors. Environmental spore exposure (from exposure to construction activity) has also led to clinical cases of mucormycosis. Other cases have been reported in patients with traumatic skin injury (eg, associated with the use of nonsterile adhesive tape or with use of tongue depressors as splints in neonates). Exposure to voriconazole, which is not active against mucormycosis, is noted to be a risk factor in patients with cancer.
No racial or sexual predilection is reported. However, most cases of mucormycosis occur in immunosuppressed adults. In a pooled review, Kline described 41 cases of rhinocerebral mucormycosis occurring in children and adolescents aged 2 months to 18 years. [10] About 49% of cases were found in patients with diabetes mellitus, and 15% of cases were found in those with leukemia. Four of the 41 children (10%) had no predisposing conditions.
Prognosis
Survival rates largely depend on early diagnosis and treatment of mucormycosis as well as resolution of the patient's underlying condition. If the disease progresses and if the underlying condition remains uncontrolled, death usually ensues. The overall mortality rate in adults is 50%, though survival rates higher than this have been reported.
Mortality depends on the site of infection. Higher mortality of 80% is observed among patients with disseminated disease to the central nervous system. [11] Mortality is also high among patients with gastrointestinal manifestations, as the diagnosis is often made late because of a nonspecific presentation. [12] Pulmonary mucormycosis carries a mortality rate of 57.1%; however, mortality rates have improved over time owing to a combination of medical and surgical treatment. [13]
In neonates, these invasive fungal infections can be rapidly fatal; the time from clinical symptoms to death is in the range of 6 to 42 days.
Because the disease is rare and because therapy is not standardized, no studies aid in predicting patient outcomes.
-
Black eschar on the skin of an immunocompromised patient.
-
Mucormycosis with broad, aseptate hyphae (hematoxylin and eosin, original magnification ×40).
-
Angioinvasion (hematoxylin and eosin, original magnification ×10).
-
Perineural invasion (hematoxylin and eosin, original magnification ×20).