Background
Retinoblastoma is the most common primary ocular malignancy (eye cancer) of childhood.
Peter Pawius of Amsterdam provided the first description of a tumor resembling retinoblastoma. He wrote of a malignancy invading the orbit, the temporal region, and the cranium, a picture now strongly suggestive of untreated retinoblastoma. The tumor was described as filled with a "substance similar to brain tissue mixed with thick blood and like crushed stone." [1]
In 1805, William Hey coined the term fungus haematodes, which he used to describe a fungating mass affecting the globe of the eye and destroying its internal organization. [2]
In 1809, the Scottish surgeon James Wardrop pieced together the random isolated facts and observations of previous authors. Despite not having a microscope at his disposal, his meticulous dissection and astute interpretations of some of these eyes led him to conclude that in most instances, the tumor arose from the retina. Wardrop documented the extension of the tumor to the optic nerve and brain. Later, he described metastasis to different parts of the body. [3]
In 1836, Langenbech, Robin, and Nystin of Paris confirmed by microscopic studies that the tumor definitely arose from the retina.
In 1864, Virchow named the tumor a glioma of the retina, supporting glial cells as the cell of origin of the tumor. [4]
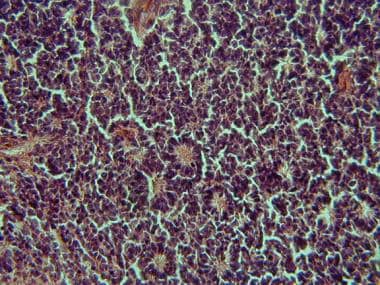
In 1891, Flexner of Johns Hopkins was first to notice rosettes within the tumor (shown in the image below). [5] A few years later in 1897, Wintersteiner concurred with Flexner and proposed the name neuroepithelioma noting its resemblance to rods and cones and traced one tumor to the photoreceptor cell layer. [6] Presently, their names are attached to these rosettes.
Most cells comprising the tumor histologically resemble the cells of an undifferentiated retina of the embryo called retinoblasts. This resemblance prompted Verhoeff to coin the term retinoblastoma, [7] which later was adopted by the American Ophthalmological Society in 1926 as a general term for this entity.
In 1970, Tso and colleagues established that the tumor arises from photoreceptor precursors. [8, 9]
In October of 2007, a team of investigators at St. Jude Children's Research Hospital (Memphis, Tenn) claimed to have identified the specific cell that gives rise to retinoblastoma. [10] The major importance of this discovery is the idea that retinoblastoma can arise from fully matured nerves in the retina called horizontal interneurons, disproving the long-held scientific principle that fully formed, mature nerves cannot multiply like young immature cells.
Pathophysiology
The most widely held concept of histogenesis of retinoblastoma holds that it generally arises from a multipotential precursor cell (mutation in the long arm of chromosome 13 band 13q14) that could develop into almost any type of inner or outer retinal cell. Intraocularly, it exhibits a variety of growth patterns, which have been described as outlined below. (See Causes for more information.)
Endophytic growth
Endophytic growth occurs when the tumor breaks through the internal limiting membrane and has an ophthalmic appearance of a white-to-cream mass showing either no surface vessels or small irregular tumor vessels. This growth pattern is typically associated with vitreous seeding, wherein small fragments of tissue become separated from the main tumor. In some instances, vitreous seeding may be extensive and allow tumor cells to be visible as spheroid masses floating in the vitreous and anterior chamber, simulating endophthalmitis or iridocyclitis, and obscuring the primary mass. Secondary deposits or seeding of tumor cells into other areas of the retina may be confused with multicentric tumors.
Exophytic growth
Exophytic growth occurs in the subretinal space. This growth pattern is often associated with subretinal fluid accumulation and retinal detachment. The tumor cells may infiltrate through the Bruch membrane into the choroid and then invade either blood vessels or ciliary nerves or vessels. Retinal vessels are noted to increase in caliber and tortuosity as they overlie the mass.
Diffuse infiltrating growth
This is a rare subtype comprising 1.5% of all retinoblastomas. It is characterized by a relatively flat infiltration of the retina by tumor cells but without a discrete tumor mass. The obvious white mass seen in typical retinoblastoma rarely occurs. It grows slowly compared with typical retinoblastoma.
Epidemiology
Frequency
United States
An estimated 250-500 new cases of retinoblastoma occur in the United States yearly.
International
Worldwide, the incidence of retinoblastoma is recorded to be about 11 cases per million children younger than 5 years. A more commonly used estimate is 1 case of retinoblastoma per 18,000-30,000 live births, depending on the country.
In the Philippines, unpublished reports have estimated the incidence to be more than 1 case of retinoblastoma per 18,000 live births.
Mortality/Morbidity
Survival rates for patients with retinoblastoma range from a reported 86-92%. However, these figures must be kept in the context of the retinoblastoma cancers. In actuality, the survival rate drops with each decade of life for patients with the genomic mutation.
The genomic mutation is a gene mutation within every cell of the individual's body. These patients typically present with either bilateral disease or unilateral-multifocal (one eye with multiple distinctly separate tumor foci) disease. These individuals have a predisposition for developing second cancers later in life.
Mortality in these individuals is consequently much higher than rates for those with somatic mutations (ie, affecting one retinal cell only and unilateral-unifocal disease). The greatest predictor of death is extraocular extension, either directly through the sclera or via extension along the optic nerve.
Race
No racial predilection appears to exist for retinoblastoma.
No difference in incidence exists among blacks and whites.
Sex
Studies show no significant difference in the incidence of retinoblastoma by sex for children aged 0-14 years.
The estimated boy-to-girl ratio is reportedly 1.12:1.
Age
Retinoblastoma is diagnosed in patients at an average of 18 months, with 90% of cases diagnosed in patients younger than 5 years.
Children who are affected bilaterally are diagnosed at an average age of 13 months, while patients with unilateral retinoblastoma are diagnosed at an average age of 24 months.
When a known family history of retinoblastoma exists, patients with bilateral retinoblastoma are diagnosed at an average age of 11 months.
A few cases of retinoblastoma in adults (aged 20 y and older) have been reported in the literature. Some theorize that these lesions arise from a previously existing retinocytoma that underwent malignant transformation.
Prognosis
In a study of 69 adult survivors of retinoblastoma assessed at an average of 31 years after diagnosis, Brinkman et al found that subjects performed within normative expectations on various measures of verbal intelligence, attention, memory, processing speed, and executive functioning; they performed above expectations in nonverbal reasoning abilities and ability to learn new information and below expectations in fine motor dexterity. [11, 12] Survivors reported more problems with working memory and task completion than adults of similar age did.
Patients who had had bilateral disease performed significantly better than those with unilateral disease on measures of verbal learning and short- and long-term verbal memory. [12] Diagnosis at less than 1 year of age was associated with better performance on a number of verbal domains, Total brain radiation exposure was negatively correlated with performance on measures of verbal learning and memory.
The prognosis in retinoblastoma is good where prompt medical care is available. The overall survival rate of retinoblastoma in the United States and Great Britain is presently greater than 85%.
The cure rate is almost 90% if the optic nerve is not involved and enucleation is performed before the tumor passes through the lamina cribrosa.
Survival rates decrease to 60% if the tumor extends beyond the lamina cribrosa even if the cut end of the nerve is free of tumor cells.
Survival rates decrease to less than 20% if the tumor cells are found at the surgical transection sight.
Death occurs secondary to intracranial extension. Treatment with EBRT results in an 85% cure rate.
Visual preservation occurs in 90% of children with group I and II disease (Reese-Ellsworth classification); 30-40% for group IV and 10-15% for patients with advanced group V disease.
Of patients previously treated with EBRT, 60% require further therapy with cryotherapy or photocoagulation.
Of patients requiring treatment with EBRT, 20% eventually require enucleation.
Patient Education
Genetic counseling for retinoblastoma
In 1979, Vogel published his review on the genetics of retinoblastoma in the Journal of Human Genetics. He reviewed the likelihood for the recurrence of retinoblastoma in close relatives of a patient with the disease, based on clinical criteria, as shown below. It is the physician's responsibility to inform the patient's family that retinoblastoma can be hereditary. The methods for screening and estimation of risks are highly improved with use of molecular genetics techniques, although this sometimes can prove to be very expensive.
 Genetic counseling for retinoblastoma. (This table is modified from Vogel F. Genetics of retinoblastoma. Hum Genet 1979; 52:1.)
Genetic counseling for retinoblastoma. (This table is modified from Vogel F. Genetics of retinoblastoma. Hum Genet 1979; 52:1.)
Normal individuals have 2 copies of the retinoblastoma gene, 1 coming from each parent. However, in patients with retinoblastoma, one copy of the gene is inactivated by an initial mutation.
When the initial mutation arises from a somatic cell (retinal), the patient has the nonhereditary type of retinoblastoma and the relatives have a low risk for the disease. These individuals have 1 abnormal gene in all their cells, and the mutation in the other gene (in the retinal cell) allows the expression of the tumor.
When the initial mutation arises from the germline, the patient has the hereditary type of retinoblastoma and the relatives of the patient have a significant risk for retinoblastoma. In these individuals, both mutations occur only in the retinal cell that has become malignant.
-
Classic histologic finding of retinoblastoma (Flexner-Wintersteiner rosettes)
-
Retinoblastoma, intraocular stage (leukocoria). History: NB, 1-year-old male from Quezon Province, Philippines, with chief complaint of opacity, left eye. Born full-term spontaneous vaginal delivery (FTSVD) to a 27-year-old gravida 3, para 2 (2002) at home. Four months prior to admission (PTA), opacity was noted in the left eye (no consultation/medications). Five days PTA, consultation with an ophthalmologist. Examination: (+) leukocoria with visual acuity of central, steady, and maintained fixation on right eye, (-) dazzle on left eye; (+) Marcus Gunn (MG) reflex. Diagnostics: Ocular ultrasound was performed, revealing intraocular retinoblastoma. Management: Patient underwent enucleation of left eye. Examination under anesthesia of right eye: E/N retina. Histopathology: Retinoblastoma, intraocular stage left eye.
-
Retinoblastoma, glaucomatous stage. History: AB, 2-year-old female from Marikina City, Philippines, with chief complaint of proptosis, right eye. The patient is an adopted child. Prior to admission (PTA), with child aged 6 months (time of adoption), surrogate mother noted an opacity in the right eye. No medical consultation. One year PTA, physician consultation; told AB had an "eye mass" and needed to see an ophthalmologist. No compliance. One month PTA, proptosis was noted in the right eye. Examination: Visual acuity (VA) of right eye is no light perception; VA of left eye is central, steady, and maintained fixation. Sensorium: Awake but irritable. Diagnostics: Intracranial extension on CT scan. Skeletal survey: E/N. Management: The patient underwent exenteration (right side).
-
Patient with retinoblastoma, glaucomatous stage. Intracranial extension on CT scan.
-
Patient with retinoblastoma, glaucomatous stage. Another CT scan slice, showing the intracranial extension of the tumor.
-
Retinoblastoma, extraocular stage (neglected with necrosis). History: RC, 2-year-old male with chief complaint of left orbital mass. Born full-term spontaneous vaginal delivery (FTSVD) to a gravida 3, para 2 (2001) at home. Three months prior to admission (PTA), an inward deviation of the left eye was noted. No consultation. Six months PTA, opacity in the left eye was noted. Five months PTA, proptosis of the left eye with pain and bleeding was noted. Family/Social History: Indigent family. Youngest of 3 siblings; eldest sibling had no retinoblastoma; second sibling had retinoblastoma and underwent enucleation, dying after 2 sessions of chemotherapy. A cousin passed away with retinoblastoma. Examination: Indirect ophthalmoscopy of right eye revealed a large intraocular mass occupying the inferior half of the retina. Mass on left side. Management: The patient was scheduled for exenteration, left side. The mother and child went home against medical advice; what happened to the patient is not known.
-
Status post (S/P) enucleation for retinoblastoma, right eye retinoblastoma, recurrence, right eye. History: IJ, 3-year-old male with chief complaint of right orbital mass. At age 2 months, opacity in right eye is noted. Five months prior to admission (PTA), consultation with an ophthalmologist for proptosis, right eye. Four months PTA, the patient underwent enucleation, right eye, with no alleged tumor involvement of the tumor resection margins on histopathology. One month PTA, gradually enlarging orbital mass, right side, was noted. Examination: Visual acuity right eye, not applicable (S/P enucleation); visual acuity left eye, at least 6/12 (20/40). No masses are seen in left eye on indirect ophthalmoscopy. Diagnostics: Skeletal survey showed lytic lesions on the humerus, femur, and pubic bones.
-
Retinoblastoma, intraocular stage (CT scan findings). History: 5-month-old female with chief complaint of "cat's eye reflex." Two months prior to admission (PTA), cat's eye reflex noted with outward deviation of left eye. The patient's 29-year-old mother had bilateral retinoblastoma and underwent enucleation, left eye, at age 2 years. Examination: Regressed type stage III, left eye visual acuity (+) dazzle right eye; indirect ophthalmoscopy (+) mass nasal retina with seeding, multiple tumors in peripheral retina, left eye. E/N Retina: Right eye. Management: The patient underwent enucleation, left eye. Examination under anesthesia of right eye: E/N. Histopathology: Retinoblastoma, intraocular stage, well-differentiated left eye.
-
Flexner-Wintersteiner rosettes in retinoblastoma
-
Presenting signs or symptoms in retinoblastoma. (This table is modified from Abramson DH, Frank CM, Susman M, et al. Presenting signs of retinoblastoma. J Pediatr 1998 Mar; 132(3 Pt 1): 505-8.)
-
Reese-Ellsworth classification of retinoblastoma
-
Classic regression patterns of retinoblastoma
-
Genetic counseling for retinoblastoma. (This table is modified from Vogel F. Genetics of retinoblastoma. Hum Genet 1979; 52:1.)
-
Vitreous seeding (intraocular retinoblastoma). Courtesy of Manolette Roque, MD, Ophthalmic Consultants Philippines Co, EYE REPUBLIC Ophthalmology Clinic.
-
Reese-Ellsworth Stage V: vitreous seeding. Courtesy of Manolette Roque, MD, Ophthalmic Consultants Philippines Co, EYE REPUBLIC Ophthalmology Clinic.