Key Points
-
Individuals are increasingly self-reporting gluten sensitivity and placing themselves on a gluten-free diet outside a diagnosis of coeliac disease or IgE-mediated wheat allergy
-
This clinical entity has been termed noncoeliac gluten sensitivity (NCGS)
-
The symptoms evoked by gluten in NCGS include a constellation of intestinal and extraintestinal symptoms
-
Nongluten components of the grain can also be responsible for triggering symptoms in individuals with NCGS
-
No diagnostic biomarkers to differentiate between gluten and nongluten components currently exist; positive antigliadin antibodies support the diagnosis of NCGS but have limited sensitivity and specificity
-
Patients presenting with NCGS are a heterogeneous group and should be counselled about the uncertainties surrounding their diagnosis
Abstract
The past 5 years have seen an increase in the use of a gluten-free diet outside a diagnosis of coeliac disease or IgE-mediated wheat allergy. This trend has led to the identification of a new clinical entity termed noncoeliac gluten sensitivity (NCGS). In this Review, we discuss the evidence for NCGS as demonstrated by the results of double-blind, placebo-controlled dietary rechallenge studies. Furthermore, the characteristic phenotype of individuals with NCGS is described as well as the symptom manifestations commonly reported after gluten exposure, which include intestinal symptoms consistent with IBS, and extraintestinal symptoms such as neurological dysfunction, psychological disturbances, fibromyalgia and skin rash. Moreover, emerging evidence suggests that NCGS can be associated with organic gastrointestinal pathologies, such as IBD, in which its presence might be a reflection of severe or stricturing disease. However, NCGS is not without its controversies and uncertainties, in particular pertaining to whether it is gluten or nongluten components of the grain evoking symptoms; evidence suggests that fermentable carbohydrates, amylase trypsin inhibitors and wheat-germ agglutinin can also be responsible culprits. Finally, we discuss the novel techniques that might help diagnose NCGS in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aziz, I., Branchi, F. & Sanders, D. S. The rise and fall of gluten! Proc. Nutr. Soc. http://dx.doi.org/10.1017/S0029665115000038.
Mooney, P. D., Hadjivassiliou, M. & Sanders, D. S. Coeliac disease. BMJ 348, g1561 (2014).
Hadithi, M. et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 147, 294–302 (2007).
Rubio-Tapia, A., Hill, I. D., Kelly, C. P., Calderwood, A. H. & Murray, J. A. ACG clinical guidelines: diagnosis and management of celiac disease. Am. J. Gastroenterol. 108, 656–676 (2013).
Ludvigsson, J. F. et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 63, 1210–1228 (2014).
Hall, N. J., Rubin, G. & Charnock, A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 30, 315–330 (2009).
Lee, A. R., Ng, D. L., Zivin, J. & Green, P. H. Economic burden of a gluten-free diet. J. Hum. Nutr. Diet. 20, 423–430 (2007).
Singh, J. & Whelan, K. Limited availability and higher cost of gluten-free foods. J. Hum. Nutr. Diet. 24, 479–486 (2011).
Karajeh, M. A., Hurlstone, D. P., Patel, T. M. & Sanders, D. S. Chefs' knowledge of coeliac disease (compared to the public): a questionnaire survey from the United Kingdom. Clin. Nutr. 24, 206–210 (2005).
Addolorato, G. et al. Social phobia in coeliac disease. Scand. J. Gastroenterol. 43, 410–415 (2008).
Aziz, I. et al. Change in awareness of gluten-related disorders among chefs and the general public in the UK: a 10-year follow-up study. Eur. J. Gastroenterol. Hepatol. 26, 1228–1233 (2014).
Ferch, C. C. & Chey, W. D. Irritable bowel syndrome and gluten sensitivity without celiac disease: separating the wheat from the chaff. Gastroenterology 142, 664–666 (2012).
Spence, D. Bad medicine: food intolerance. BMJ 346, f529 (2013).
Tanpowpong, P. et al. Coeliac disease and gluten avoidance in New Zealand children. Arch. Dis. Child. 97, 12–16 (2012).
Rubio-Tapia, A., Ludvigsson, J. F., Brantner, T. L., Murray, J. A. & Everhart, J. E. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 107, 1538–1544 (2012).
Aziz, I. et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 26, 33–39 (2014).
Lis, D., Stellingwerff, T., Shing, C. M., Ahuja, K. D. & Fell, J. W. Exploring the popularity, experiences and beliefs surrounding gluten-free diets in non-coeliac athletes. Int. J. Sport Nutr. Exerc. Metab. 25, 37–45 (2015).
Golley, S., Corsini, N., Topping, D., Morell, M. & Mohr, P. Motivations for avoiding wheat consumption in Australia: results from a population survey. Public Health Nutr. 18, 490–499 (2015).
Mardini, H. E., Westgate, P. & Grigorian, A. Y. Racial differences in the prevalence of celiac disease in the US population: National Health and Nutrition Examination Survey (NHANES) 2009–2012. Dig. Dis. Sci. 60, 1738–1742 (2015).
Cooper, B. T., Holmes, G. K., Ferguson, R., Thompson, R. & Cooke, W. T. Proceedings: Chronic diarrhoea and gluten sensitivity. Gut 17, 398 (1976).
Ellis, A. & Linaker, B. D. Non-coeliac gluten sensitivity? Lancet 1, 1358–1359 (1978).
Cooper, B. T. et al. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 79, 801–806 (1980).
Falchuk, Z. M. Gluten-sensitive diarrhea without enteropathy: fact of fancy? Gastroenterology 79, 953–955 (1980).
Cooper, B. T. et al. “Gluten-sensitive diarrhea without evidence of celiac disease”. Gastroenterology 81, 192–194 (1981).
Verdu, E. F., Armstrong, D. & Murray, J. A. Between celiac disease and irritable bowel syndrome: the “no man's land” of gluten sensitivity. Am. J. Gastroenterol. 104, 1587–1594 (2009).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4 (2012).
Böhn, L., Störsrud, S., Törnblom, H., Bengtsson, U. & Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 108, 634–641 (2013).
Lind, R. et al. Subjective health complaints and modern health worries in patients with subjective food hypersensitivity. Dig. Dis. Sci. 50, 1245–1251 (2005).
Berstad, A., Undseth, R., Lind, R. & Valeur, J. Functional bowel symptoms, fibromyalgia and fatigue: a food-induced triad? Scand. J. Gastroenterol. 47, 914–919 (2012).
Boettcher, E. & Crowe, S. E. Dietary proteins and functional gastrointestinal disorders. Am. J. Gastroenterol. 108, 728–736 (2013).
Young, E., Stoneham, M. D., Petruckevitch, A., Barton, J. & Rona, R. A population study of food intolerance. Lancet 343, 1127–1130 (1994).
Carroccio, A. et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am. J. Gastroenterol. 107, 1898–1906 (2012).
Biesiekierski, J. R. et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 106, 508–514 (2011).
Carroccio, A., Mansueto, P., D'Alcamo, A. & Iacono, G. Non-celiac wheat sensitivity as an allergic condition: personal experience and narrative review. Am. J. Gastroenterol. 108, 1845–1852 (2013).
Sapone, A. et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 10, 13 (2012).
Catassi, C. et al. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients 5, 3839–3853 (2013).
Biesiekierski, J. R. et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145, 320–328 (2013).
Francavilla, R. et al. Clinical, serologic, and histologic features of gluten sensitivity in children. J. Pediatr. 164, 463–467 (2014).
Volta, U., Bardella, M. T., Calabrò, A., Troncone, R. & Corazza, G. R. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 12, 85 (2014).
Volta, U. et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 46, 680–685 (2012).
Tavakkoli, A., Lewis, S. K., Tennyson, C. A., Lebwohl, B. & Green, P. H. Characteristics of patients who avoid wheat and/or gluten in the absence of celiac disease. Dig. Dis. Sci. 59, 1255–1261 (2014).
Carroccio, A. et al. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: a prospective observation study. BMC Med. 12, 230 (2014).
Kabbani, T. A. et al. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am. J. Gastroenterol. 109, 741–746 (2014).
Troncone, R. et al. In siblings of celiac children, rectal gluten challenge reveals gluten sensitization not restricted to celiac HLA. Gastroenterology 111, 318–324 (1996).
Caio, G., Volta, U., Tovoli, F. & De Giorgio, R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 14, 26 (2014).
Sapone, A. et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 9, 23 (2011).
Sapone, A. et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 152, 75–80 (2010).
Volta, U. & De Giorgio, R. New understanding of gluten sensitivity. Nat. Rev. Gastroenterol. Hepatol. 9, 295–299 (2012).
Villanacci, V., Lanzini, A., Lanzarotto, F. & Ricci, C. Observations on the paper of Carroccio. et al. “Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity”. Am. J. Gastroenterol. 108, 619–620 (2013).
Carroccio, A., Mansueto, P., Tripodo, C. & Florena, A. M. Response to Villanacci. et al. Am. J. Gastroenterol. 108, 620 (2013).
Volta, U., Caio, G., Tovoli, F. & De Giorgio, R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell. Mol. Immunol. 10, 383–392 (2013).
Brottveit, M. et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 108, 842–850 (2013).
Vazquez-Roque, M. I. et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144, 903–911 (2013).
Hollon, J. et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 7, 1565–1576 (2015).
Bucci, C. et al. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin. Gastroenterol. Hepatol. 11, 1294–1299 (2013).
Verdu, E. F. et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G217–G225 (2008).
Aziz, I. & Hadjivassiliou, M. Coeliac disease: noncoeliac gluten sensitivity—food for thought. Nat. Rev. Gastroenterol. Hepatol. 11, 398–399 (2014).
Hadjivassiliou, M. et al. Gluten sensitivity: from gut to brain. Lancet Neurol. 9, 318–330 (2010).
Hadjivassiliou, M. et al. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 80, 1740–1745 (2013).
Hadjivassiliou, M., Davies-Jones, G. A., Sanders, D. S. & Grünewald, R. A. Dietary treatment of gluten ataxia. J. Neurol. Neurosurg. Psychiatry 74, 1221–1224 (2003).
Hadjivassiliou, M. et al. Neuropathy associated with gluten sensitivity. J. Neurol. Neurosurg. Psychiatry 77, 1262–1266 (2006).
Hadjivassiliou, M. et al. Dietary treatment of gluten neuropathy. Muscle Nerve 34, 762–766 (2006).
Hadjivassiliou, M. et al. Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology 56, 385–388 (2001).
Peters, S. L., Biesiekierski, J. R., Yelland, G. W., Muir, J. G. & Gibson, P. R. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment. Pharmacol. Ther. 39, 1104–1112 (2014).
Aziz, I., Hadjivassiliou, M. & Sanders, D. S. Editorial: Noncoeliac gluten sensitivity—a disease of the mind or gut? Aliment. Pharmacol. Ther. 40, 113–114 (2014).
Brottveit, M. et al. Absence of somatization in non-coeliac gluten sensitivity. Scand. J. Gastroenterol. 47, 770–777 (2012).
Kalaydjian, A. E., Eaton, W., Cascella, N. & Fasano, A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr. Scand. 113, 82–90 (2006).
Dickerson, F. et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol. Psychiatry 68, 100–104 (2010).
Cascella, N. G. et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr. Bull. 37, 94–100 (2011).
Cascella, N. G. et al. Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr. Bull. 39, 867–871 (2013).
Isasi, C. et al. Fibromyalgia and non-celiac gluten sensitivity: a description with remission of fibromyalgia. Rheumatol. Int. 34, 1607–1612 (2014).
Rodrigo, L., Blanco, I., Bobes, J. & de Serres, F. J. Effect of one year of a gluten-free diet on the clinical evolution of irritable bowel syndrome plus fibromyalgia in patients with associated lymphocytic enteritis: a case-control study. Arthritis Res. Ther. 16, 421 (2014).
Michaëlsson, G. et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br. J. Dermatol. 142, 44–51 (2000).
Biesiekierski, J. R., Newnham, E. D., Shepherd, S. J., Muir, J. G. & Gibson, P. R. Characterization of adults with a self-diagnosis of nonceliac gluten sensitivity. Nutr. Clin. Pract. 29, 504–509 (2014).
Leffler, D. et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 62, 996–1004 (2013).
Tortora, R. et al. In vitro gliadin challenge: diagnostic accuracy and utility for the difficult diagnosis of celiac disease. Am. J. Gastroenterol. 107, 111–117 (2012).
Aziz, I., Evans, K. E., Hopper, A. D., Smillie, D. M. & Sanders, D. S. A prospective study into the aetiology of lymphocytic duodenosis. Aliment. Pharmacol. Ther. 32, 1392–1397 (2010).
Molina-Infante, J., Santolaria, S., Fernandez-Bañares, F., Montoro, M. & Esteve, M. Lymphocytic enteropathy, HLA-DQ2/DQ8 genotype and wheat-dependent symptoms: non-celiac wheat sensitivity or Marsh I celiac disease? Am. J. Gastroenterol. 108, 451 (2013).
Carroccio, A. & Mansueto, P. Response to Molina-Infante. et al. Am. J. Gastroenterol. 108, 451–452 (2013).
Carroccio, A. et al. Antiendomysium antibodies assay in the culture medium of intestinal mucosa: an accurate method for celiac disease diagnosis. Eur. J. Gastroenterol. Hepatol. 23, 1018–1023 (2011).
Not, T. et al. Cryptic genetic gluten intolerance revealed by intestinal antitransglutaminase antibodies and response to gluten-free diet. Gut 60, 1487–1493 (2011).
Aziz, I., Hadjivassiliou, M. & Sanders, D. S. Self-reported gluten sensitivity: an international concept in need of consensus? Am. J. Gastroenterol. 109, 1498–1499 (2014).
Coburn, J. A. et al. Human leukocyte antigen genetics and clinical features of self-treated patients on a gluten-free diet. J. Clin. Gastroenterol. 47, 828–833 (2013).
Kaukinen, K. et al. Intolerance to cereals is not specific for coeliac disease. Scand. J. Gastroenterol. 35, 942–946 (2000).
Campanella, J. et al. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand. J. Gastroenterol. 43, 1311–1314 (2008).
Catassi, C. et al. How the diagnosis of non-celiac gluten sensitivity (NCGS) should be confirmed: The Salerno Experts' Criteria. Nutrients (in press).
Nijeboer, P., Bontkes, H. J., Mulder, C. J. & Bouma, G. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J. Gastrointestin. Liver Dis. 22, 435–440 (2013).
Shepherd, S. J., Parker, F. C., Muir, J. G. & Gibson, P. R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 6, 765–771 (2008).
Murray, K. et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 109, 110–119 (2014).
Halmos, E. P., Power, V. A., Shepherd, S. J., Gibson, P. R. & Muir, J. G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75 (2014).
Staudacher, H. M., Irving, P. M., Lomer, M. C. & Whelan, K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 11, 256–266 (2014).
Biesiekierski, J. R. et al. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 24, 154–176 (2011).
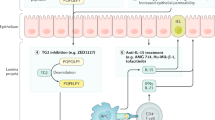
Junker, Y. et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 209, 2395–2408 (2012).
Tilg, H., Koch, R. & Moschen, A. R. Proinflammatory wheat attacks on the intestine: alpha-amylase trypsin inhibitors as new players. Gastroenterology 144, 1561–1563 (2013).
Dalla Pellegrina, C. et al. Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 237, 146–153 (2009).
Miyake, K., Tanaka, T. & McNeil, P. L. Lectin-based food poisoning: a new mechanism of protein toxicity. PLoS ONE 2, e687 (2007).
Carroccio, A., Rini, G. & Mansueto, P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 146, 320–321 (2014).
Di Sabatino, A. et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebo-controlled, cross-over trial. Clin. Gastroenterol. Hepatol. http://dx.doi.org/10.1016/j.cgh.2015.01.029.
Sanders, D. S. et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet 358, 1504–1508 (2001).
Sanders, D. S. et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur. J. Gastroenterol. Hepatol. 15, 407–413 (2003).
Cash, B. D. et al. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology 141, 1187–1193 (2011).
Wahnschaffe, U., Ullrich, R., Riecken, E. O. & Schulzke, J. D. Celiac disease-like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology 121, 1329–1338 (2001).
Wahnschaffe, U., Schulzke, J. D., Zeitz, M. & Ullrich, R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 5, 844–850 (2007).
Ludvigsson, J. F. et al. The Oslo definitions for coeliac disease and related terms. Gut 62, 43–52 (2013).
Vazquez-Roque, M. I. et al. HLA-DQ genotype is associated with accelerated small bowel transit in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 23, 481–487 (2011).
Herfarth, H. H., Martin, C. F., Sandler, R. S., Kappelman, M. D. & Long, M. D. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 20, 1194–1197 (2014).
Aziz, I., Branchi, F., Pearson, K., Priest, J. & Sanders, D. S. A study evaluating the bidirectional relationship between inflammatory bowel disease and self-reported non-celiac gluten sensitivity. Inflamm. Bowel Dis. 21, 847–853 (2015).
Carroccio, A. et al. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 8, 254–260 (2010).
Carroccio, A. et al. A comparison between two different in vitro basophil activation tests for gluten- and cow's milk protein sensitivity in irritable bowel syndrome (IBS)-like patients. Clin. Chem. Lab. Med. 51, 1257–1263 (2013).
Carroccio, A. et al. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 9, 965–971 (2011).
Valerii, M. C. et al. Responses of peripheral blood mononucleated cells from non-celiac gluten sensitive patients to various cereal sources. Food Chem. 176, 167–174 (2015).
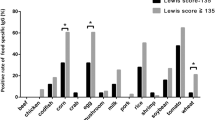
Fritscher-Ravens, A. et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 147, 1012–1020 (2014).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects in the production of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Aziz, I., Hadjivassiliou, M. & Sanders, D. The spectrum of noncoeliac gluten sensitivity. Nat Rev Gastroenterol Hepatol 12, 516–526 (2015). https://doi.org/10.1038/nrgastro.2015.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.107