Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coagulants
2.2. Water Samples Used for Coagulation
2.3. Experimental Procedure of Coagulation
2.4. Analytical Methods
3. Results and Discussion
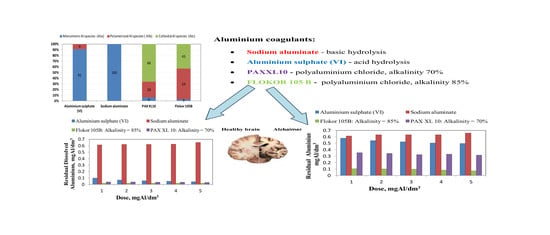
3.1. Coagulant Properties
3.2. Influence of Type and Dose of Aluminium Coagulants on the Concentration of Other Aluminium
4. Conclusions
Funding
Conflicts of Interest
References
- Bachir, M.; Brakchi, S.; Azzouz, A. Risk of residual aluminum in treated waters with aluminum sulphate. Adv. Res. 2016, 6, 1–8. [Google Scholar]
- Krupińska, I. Effect of temperature and pH on the effectiveness of pollutant removal from groundwater in the process of coagulation. Ochrona Środowiska 2015, 37, 35–42. [Google Scholar]
- Chao, H.J.; Zhang, X.; Wang, W.; Li, D.; Ren, Y.; Kang, J.; Liu, D. Evaluation of carboxymethylpullulan-AlCl3 as a coagulant for water treatment: A case study with kaolin. Water Environ. Res. 2019, 1, 1–8. [Google Scholar]
- D’Haese, P.C.; Douglas, G.; Verhulst, A.; Neven, E.; Behets, G.J.; Vervaet, B.A.; Finsterle, K.; Lürling, M.; Spears, B. Human health risk associated with the management of phosphorus in freshwaters using lanthanum and aluminium. Chemosphere 2019, 220, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, X.; Li, Z.; Pan, B.; Hao, Y.; Niu, Q. Aluminium-induced synaptic plasticity injury via the PHF8-H3K9me2-BDNF signalling pathway. Chemosphere 2020, 244, 1–10. [Google Scholar] [CrossRef]
- Crisponi, G.; Fanni, D.; Gerosa, C.; Nemolato, S.; Nurchi, V.M.; Crespo-Alonso, M.; Lachowicz, J.I.; Faa, G. The meaning of aluminium exposure on human health and aluminium-related diseases. Biomol. Concepts 2013, 4, 77–87. [Google Scholar] [CrossRef]
- Yue, Z.; Baoyou, S.; Yuanyuan, Z.; Mingquan, Y.; Darren, A.L.; Dongsheng, W. Deposition behavior of residual aluminum in drinking water distribution system: Effect of aluminum speciation. J. Environ. Sci-China. 2016, 42, 142–151. [Google Scholar]
- Chadwick, D.J.; Whelan, J. Aluminium and Its Role in Biology and Medicine; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Campbell, A. The potential role of aluminium in Alzheimer’s disease. Nephrol Dial. Transpl 2002, 17, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Oram, B. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals|Drinking Water Contaminants–Standards and Regulations; US EPA, Publisher: Washington DC, USA, 19 January 2017. Available online: https://water-research.net/index.
- World Health Organization. Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. 2017. Available online: https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ (accessed on 30 January 2020).
- Regulation of the Minister of Health dated December 7, 2017 amending the regulation on the quality of drinking water mean for human consumption. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20170002294/O/D20172294.pdf (accessed on 30 January 2020).
- Ruyuan, J.; Hui, X.; Weiying, X.; Xiaofang, Y.; Dongsheng, W. Influence of coagulation mechanisms on the residual aluminium—The roles of coagulant species and MW of organic matter. J. Hazard. Mater. 2015, 290, 16–25. [Google Scholar]
- Płuciennik-Koropczuk, E.; Mossetty, J. Chemical stability of water in the water supply network—preliminary research. Civil. Environ. Eng. Rep. 2018, 28, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Jakubaszek, A.; Kumanowska, P. Changes to water quality in the water supply network of Zielona Góra. Civil. Environ. Eng. Rep. 2019, 29, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Krupińska, I. Suitability of Coagulation for Treatment of Groundwater. Rocz Ochr Sr 2012, 14, 491–501. [Google Scholar]
- Krupińska, I. The impact of the oxidising agent type and coagulant type on the effectiveness of coagulation in the removal of pollutants from underground water with an increased content of organic substances. J. Environ. Eng Landsc. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Krupińska, I. The influence of aeration and type of coagulant on effectiveness in removing pollutants from groundwater in the process of coagulation. Chem Biochem Eng Q. 2016, 30, 465–475. [Google Scholar] [CrossRef]
- Krupińska, I. Removal of natural organic matter from groundwater by coagulation using prehydrolysed and non-prehydrolysed coagulants. Desalin Water Treat. 2018, 132, 244–252. [Google Scholar] [CrossRef]
- Nowacka, A.; Włodarczyk Makuła, M.; Macherzyński, B. Comparison of effectiveness of coagulation with aluminum sulfate and pre-hydrolyzed aluminum coagulants. Desalin Water Treat. 2014, 52, 3843–3851. [Google Scholar] [CrossRef]
- Nilsson, R. Residual aluminium concentration in drinking water after treatment with aluminium or iron salts. In Chemical Water and Wastewater Treatment; Hahn, H.H., Klute, R., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 1990; pp. 399–410. [Google Scholar]
- Sieliechi, J.M.; Kayem, G.J.; Sandu, I. Effect of water treatment residuals (aluminium and iron ions) on human health and drinking water distribution systems. Int.J. Conservat. Sci. 2010, 1, 175–182. [Google Scholar]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere. 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Frommell, D.M.; Feld, C.M.; Snoeyink, V.L.; Melcher, B.; Feizoulof, C. Aluminum residual control using orthophosphate. JAWWA 2004, 96, 99–109. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Yu, J.; Ni, J.; Edwards, M.; Qu, J. Enhanced coagulation with polyaluminum chlorides: Role of pH / Alkalinity and speciation. Chemosphere. 2008, 71, 1665–1673. [Google Scholar] [CrossRef]
- Yang, Z.L.; Gao, B.Y.; Yue, Q.Y.; Wang, Y. Effect of pH on the coagulation performance of Al-based coagulants and residual aluminum speciation during the treatment of humic acid-kaolin synthetic water. J. Hazard. Mater. 2010, 178, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, B.; Wang, Y.; Wang, Q.; Yue, Q. Aluminum fractions in surface water from reservoirs by coagulation treatment with polyaluminum chloride (PAC): Influence of initial pH and OH-/Al3+ ratio. Chem. Eng. J. 2011, 170, 107–113. [Google Scholar] [CrossRef]
- Gumińska, J. Effect of Changes in Al Speciation on the Efficiency of Water Treatment with Pre-hydrolyzed Coagulants. Ochrona Środowiska 2011, 33, 17–21. [Google Scholar]
- Hussain, S.; Van Leeuwen, J.; Chow, C.; Beecham, S.; Kamruzzaman, M.; Wang, D.; Drikas, M.; Aryal, R. Removal of organic contaminants from river and reservoir waters by three different aluminum-based metal salts: Coagulation adsorption and kinetics studies. Chem. Eng. J. 2013, 225, 394–405. [Google Scholar] [CrossRef]
- Kimura, M.; Motsui, Y.; Kendo, K.; Ishikawa, T.B. Minimizing residual aluminum concentration in treated water by tailoring properties of polyaluminum coagulants. Water Res. 2013, 47, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Machi, J.; Mołczan, J. Methods for natural organic matter characterization in water taken and treated for human consuption. Ochrona Środowiska 2016, 38, 25–32. [Google Scholar]
- Szlachta, M.; Adamski, W. Assessing the efficiency of Natural Organic Matter (NOM) removal from water by coagulation. Ochrona Środowiska 2008, 30, 9–11. [Google Scholar]
- Wang, W.; Wanga, W.; Fan, Q.; Wang, Y.; Qiao, Z.; Wanga, X. Effects of UV radiation on humic acid coagulation characteristics in drinking water treatment processes. Chem. Eng. J. 2014, 256, 137–143. [Google Scholar] [CrossRef]
- Karanfil, T.; Schlautman, M.A.; Erdogan, I. Survey of DOC and UV measurement practices with implications for SUVA determination. J. Am. Water Works Ass. 2002, 94, 68–80. [Google Scholar] [CrossRef]
- Manufacturer’s specification (Coagulants: Sodium aluminate, aluminium sulphate (VI), PAX XL10, Flokor 105B). Available online: http://dempol.com.pl/koagulanty/http://www.kemipol.com.pl/oferta/uzdatnianie-wody/ (accessed on 30 January 2020).
- Zhou, W.; Gao, B.; Yue, Q.; Liu, L.; Wang, Y. Al-Ferron kinetics and quantitative calculation of Al(III) species in polyaluminum chloride coagulants. Colloid Surface A. 2006, 278, 235–240. [Google Scholar] [CrossRef]
- Wang, D.; Tang, H.; Gregory, J. Relative importance of charge neutralization and precipitation on coagulation of Kaolin with PACl: Effect of sulfate ion. Environ. Sci. Technol. 2002, 36, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Edzwald, J.K.; Tobiason, J.E. Enhanced versus optimized multiple objective coagulation. In Chemical Water and Wastewater Treatment V; Hahn, H., Hoffmann, E., Odegaard, H., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 1998; pp. 113–124. [Google Scholar]
- Matsui, Y.; Yuasa, A. Dynamic analysis of coagulation with alum and PACl. J. Am. Water Works Ass. 1998, 90, 96–108. [Google Scholar] [CrossRef]
- Yan, M.; Wang, J.; Qu Ni, J.; Chow, C.W.K.; Liu, H. Mechanism of natural organic matter removal by polyaluminum chloride: Effect of coagulant particle size and hydrolysis kinetics. Water Res. 2008, 42, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Brighu, U.; Choudhary, A. Parameters affecting enhanced coagulation: A review. Environ. Technol. Rev. 2018, 7, 156–176. [Google Scholar] [CrossRef]
- Dempsey, B.A.; Ganho, R.M.; O’Melia, C.R. The coagulation of humic substances by means of aluminum salts. J. Am. Water Works Ass. 1984, 76, 141–151. [Google Scholar] [CrossRef]
- Packham, R.F. The coagulation process. II. Effect of pH on the precipitation of aluminium hydroxide. J. Appl. Chem. 2007, 12, 564–568. [Google Scholar] [CrossRef]
- Krupińska, I.; Płuciennik-Koropczuk, E.; Gągała, S. Residual aluminium in water intended for human consumption. CEER 2019, 4, 248–256. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |






| Indicator | Type of Coagulant | |||
|---|---|---|---|---|
| Aluminium Sulphate (VI) | Sodium Aluminate | PAX XL10 | Flokor 105B | |
| Alkalinity ratio, r = [OH−]/[Al3+] | - | - | 2.10 | 2.55 |
| Alkalinity (Z), % | - | - | 70 ± 7 | 85 ± 5 |
| pH, - | 2.4 ± 0.5 | 12.5 ± 0.5 | 2.50 ± 0.12 | 4.20 ± 0.50 |
| Al3+, % | 4.2 ± 0.2 | 9.5 ± 0.5 | 5.0 ± 0.1 | 7.0 ± 0.5 |
| Fetot, % | <0.007 | <0.003 | - | - |
| Indicator | Unit | Value | ||
|---|---|---|---|---|
| Minimum | Average | Maximum | ||
| pH | - | 7.60 | - | 7.84 |
| Alkalinity | mmol/dm3 | 3.55 | 3.65 | 3.80 |
| Turbidity | NTU | 10.6 | 12.8 | 15.0 |
| Colour | mgPt/dm3 | 6 | 11 | 16 |
| TOC | mgC/dm3 | 6.781 | 6.832 | 7.282 |
| DOC | mgC/dm3 | 6.264 | 6.576 | 6.888 |
| Aluminium total | mgAl/dm3 | 0.003 | 0.012 | 0.020 |
| Aluminum colloidal | mgAl/dm3 | 0.000 | 0.002 | 0.004 |
| Aluminum dissolved | mgAl/dm3 | 0.003 | 0.010 | 0.016 |
| Zeta Potential | mV | −14.00 | −14.00 | −14.00 |
| Iron total | mgFe/dm3 | 0.953 | 1.269 | 1.584 |
| Iron (II) | mgFe/dm3 | 0.070 | 0.077 | 0.083 |
| UV254 | m−1 | 14.25 | 15.37 | 16.49 |
| SUVA [UV254/DOC] | m2/gC | 2.18 | 2.34 | 2.49 |
| Aluminium Species | Aluminium Sulphate (VI) | Sodium Aluminate | PAX XL10 | Flokor 105B |
|---|---|---|---|---|
| Monomeric Al species (Ala), % | 91 | 100 | 6 | 3 |
| Polymerized Al species (Alb), % | 9 | 0 | 28 | 54 |
| Colloidal Al species (Alc),% | 0 | 0 | 66 | 43 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupińska, I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules 2020, 25, 641. https://doi.org/10.3390/molecules25030641
Krupińska I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules. 2020; 25(3):641. https://doi.org/10.3390/molecules25030641
Chicago/Turabian StyleKrupińska, Izabela. 2020. "Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health" Molecules 25, no. 3: 641. https://doi.org/10.3390/molecules25030641
APA StyleKrupińska, I. (2020). Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules, 25(3), 641. https://doi.org/10.3390/molecules25030641