- Home
- Publications
- PAGES Magazine
- Snow Petrel Stomach-oil Deposits As a New Biological Archive of Antarctic Sea Ice
Snow petrel stomach-oil deposits as a new biological archive of Antarctic sea ice
McClymont EL, Bentley MJ, Hodgson DA, Spencer-Jones CL, Wardley T, West MD, Croudace IW, Berg S, Gröcke DR, Kuhn G, Jamieson SSR, Sime LC & Phillips RA
Past Global Changes Magazine
30(2)
82-83
2022
Erin L. McClymont![]() 1, M.J. Bentley1, D.A. Hodgson1,2, C.L. Spencer-Jones1, T. Wardley1, M.D. West1, I.W. Croudace3, S. Berg4, D.R. Gröcke5, G. Kuhn6, S.S.R. Jamieson1, L.C. Sime2 and R.A. Phillips2
1, M.J. Bentley1, D.A. Hodgson1,2, C.L. Spencer-Jones1, T. Wardley1, M.D. West1, I.W. Croudace3, S. Berg4, D.R. Gröcke5, G. Kuhn6, S.S.R. Jamieson1, L.C. Sime2 and R.A. Phillips2
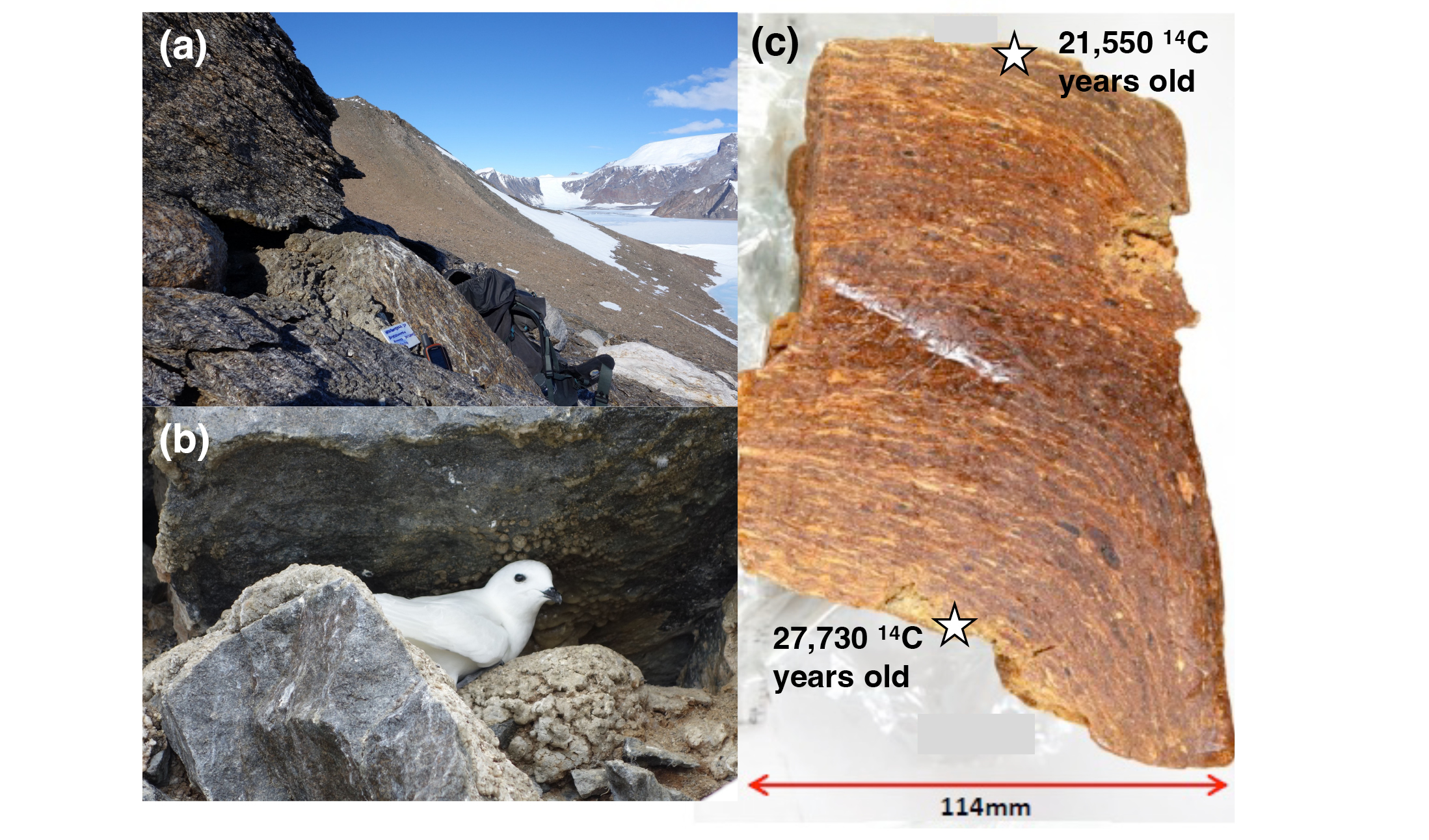
Where snow petrels forage is predominantly a function of sea ice. They spit stomach oil in defence, and accumulated deposits at nesting sites are providing new opportunities to reconstruct their diet, and, in turn, the sea-ice environment over past millennia.
Antarctic sea ice is important for the climate system, because it influences planetary albedo, ocean–atmosphere heat and gas exchange, and the formation of intermediate and deep water masses which store heat and carbon (Wang et al. 2022). Sea ice also supports unique ecosystems, with the productivity of different species linked to the seasonal and spatial changes in light and nutrient availability (Meredith et al. 2019). Antarctic sea ice is complex, including seasonal and multiyear sea ice of varying albedo and thickness. The sea ice is also broken by open waters which range in scale from small and ephemeral leads (<1 m to ~1 km) to more persistent polynyas (~1000–400,000 km2), which can drive high primary productivity and ocean–atmosphere heat and gas exchange (Arrigo and van Dijken 2003).
The instrumental record has been characterized by regionally variable trends in Antarctic sea-ice extent since the 1970s, but with no overall trend until a decrease began in 2015 (Wang et al. 2022). Understanding what this means for future sea-ice–climate interactions is complicated: the short instrumental records and the challenges of modeling such a complex environment mean we have low confidence projecting sea-ice extent this century (Fox-Kemper et al. 2021).
Paleoclimate archives have extended the instrumental record back through time. Past sea-ice margins and seasonal sea-ice zones have been mapped, largely drawing on fossil diatom assemblages and geochemical markers in marine sediments (Xiao et al. 2016; Crosta et al. 2022). Polynyas have been indicated by changing bottom current flows (Sprenk et al. 2014) or intervals of high biological productivity (Smith et al. 2010). Marine aerosols in ice cores have also revealed regional-scale changes to sea-ice extent and biological productivity (Goto-Azuma et al. 2019).
Relatively little is known about the past properties of the sea ice away from the margins, and even less about the sea-ice ecosystem, beyond those organisms preserved in the microfossil record. However, analyses of stomach-oil deposits generated by snow petrels (Pagodroma nivea) at their nesting sites above the Antarctic Ice Sheet have provided new insights: radiocarbon dating has confirmed that these seabirds were present onshore during the Last Glacial Maximum (~23–19 kyr before present (BP)), even when sea-ice extent was likely doubled relative to today (Thatje et al. 2008). But where were the petrels foraging, and was their diet the same as now? What were the sea-ice conditions where they foraged, and how have these changed over time? These questions are beginning to be answered by exploiting the unique stomach-oil archives to read the climate stories.
Snow petrels as sea-ice reporters
Snow petrels are closely associated with Antarctic sea ice, where they are present year-round at the margins and in leads and polynyas (Ainley et al. 1984). During the summer breeding season, they nest in crevices, under boulders, and in scree slopes on the mountains (nunataks) which poke through the Antarctic Ice Sheet (Fig. 1). They continue to forage in the sea ice, traveling hundreds of kilometers from their nests, returning with an energy-rich stomach oil generated from partially digested krill, squid, and fish. The oil is regurgitated as a defense mechanism against predators, and can accumulate around the nest entrance over hundreds or thousands of years (Hiller et al. 1995; Berg et al. 2019; McClymont et al. 2022). The deposits contain a mixture of stomach oils, guano, feathers, and wind-blown sediments (Fig. 1).
The close association of snow petrels with the sea ice means that the deposits are both an archive of snow petrel diet and the sea-ice environment where they fed. For example, several Antarctic seabirds vary the relative contributions of krill and fish in their diet in response to changing prey availability (Fijn et al. 2012). Isolating the biochemical fingerprints of those prey allows us to reconstruct past diets, for example, identifying krill from elevated copper and specific fatty acid distributions (McClymont et al. 2022). Some deposits have also yielded climate proxies more commonly used in marine sediments, including sea-ice diatoms (Berg et al. 2019).
A new archive of sea-ice environments at the onset of the Last Glacial Maximum
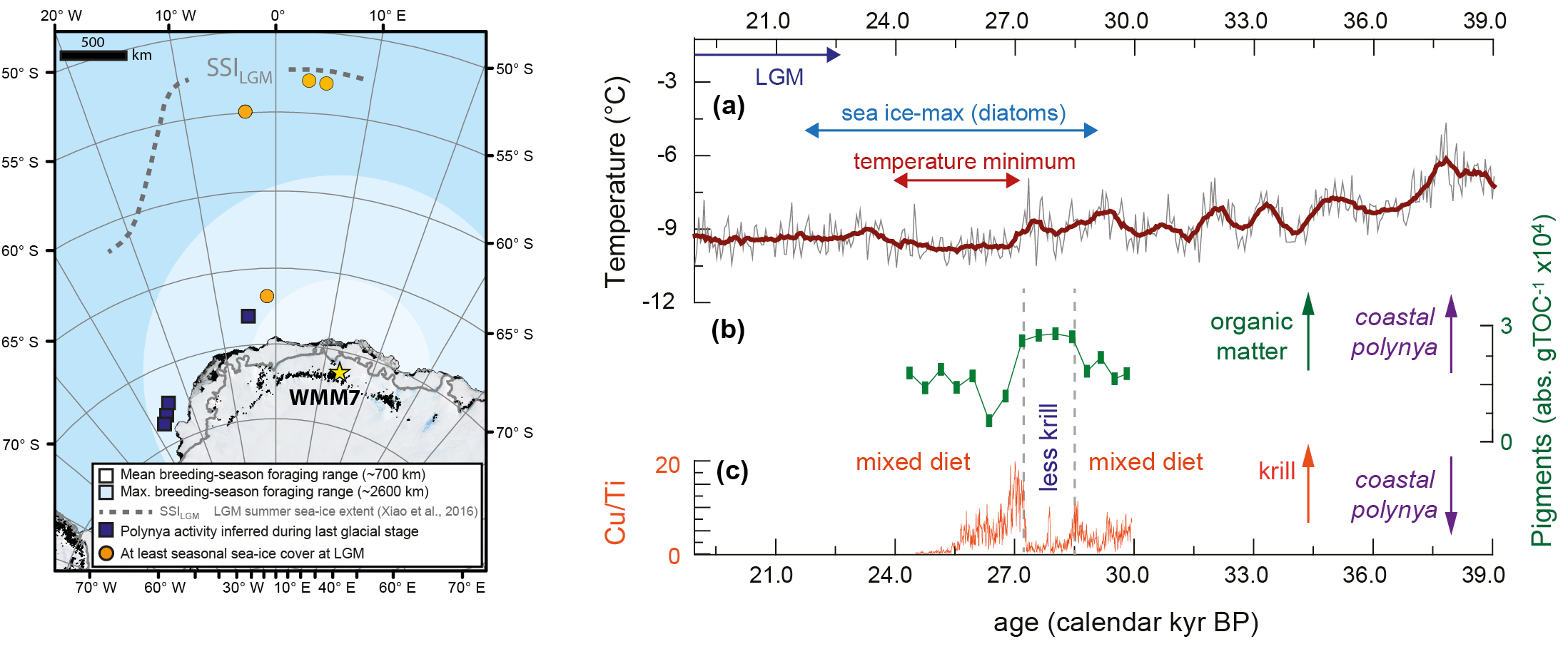
Although the global Last Glacial Maximum occurred ~23–19 kyr BP, maximum Antarctic sea-ice extent was likely reached earlier, ~29–22 kyr BP (Xiao et al. 2016; Goto-Azuma et al. 2019). We analyzed a stomach-oil deposit spanning ~30–24 kyr BP from Untersee, Dronning Maud Land. As snow petrel foraging ranges are limited by the need to return to the nest site, this deposit integrates information about sea-ice environments within ~1000 km of the coastline, in the Atlantic sector of the Southern Ocean (Fig. 2). Stable accumulation rates of the deposit suggest continuous snow petrel nest occupation even as the climate was cooling and sea ice was expanding (McClymont et al. 2022).
Using a range of proxy indicators we showed that the snow petrel diet changed through time. Overall, a mixed diet of fish, squid and krill was recorded. However, a ~1000 yr interval when krill was a minor diet component was revealed by a loss of krill fatty acids and copper (Fig. 2). The loss of krill seems likely to reflect a shift in foraging habitat to more coastal waters. But does this mean that the snow petrels were foraging at sea-ice margins which had retreated closer to the coast? This seems unlikely, as this deposit coincides with the maximum sea-ice extent, and the sea-ice margin lay far beyond the snow petrel foraging range (Fig. 2).
To resolve this conundrum, we inferred that the low-krill interval could instead reflect the opening of coastal polynyas, perhaps driven by more intense katabatic winds or a shift in the margins of the Antarctic Ice Sheet (McClymont et al. 2022). This interpretation supports the hypothesis that polynyas were important biological refugia for Antarctic ecosystems during glaciations (Thatje et al. 2008).
Outlook
Snow petrel stomach-oil deposits are revealing new information about how Antarctic seabird diets have changed through the transition to more extensive sea ice during the last glacial stage, and its subsequent retreat through the Holocene. Our results complement and extend those from marine sediments, microfossils, and ice cores, by providing regionally focussed, high-temporal-resolution records of conditions behind the sea-ice margins. By expanding our analyses to a wider network of deposits and biochemical proxies, we hope to generate new, long-term biological records of Antarctic sea ice which can be used to test and explore climate models of past, present, and future.
Acknowledgements
This research has been supported by the European Research Council H2020 (ANTSIE; grant no. 864637), the Leverhulme Trust (Research Leadership Award), and the Deutsche Forschungsgemeinschaft (DFG) priority program SPP 1158 "Antarctic Research with comparative investigations in Arctic ice areas" (BE4764/5-1).
affiliations
1Department of Geography, Durham University, UK
2British Antarctic Survey, Natural Environment Research Council, Cambridge, UK
3Ocean and Earth Science, University of Southampton, National Oceanography Centre, UK
4Institute of Geology and Mineralogy, University of Cologne, Germany
5Department of Earth Science, Durham University, UK
6Alfred Wegener Institute, Helmholtz-Center for Polar and Marine Research, Bremerhaven, Germany
contact
Erin McClymont: erin.mcclymont durham.ac.uk
durham.ac.uk
references
Ainley DG et al. (1984) Ornithol Monogr 32. American Ornithological Society, 97 pp
Arrigo KR, van Dijken GL (2003) J Geophys Res: Oceans 108: 3271
Berg S et al. (2019) Geochem Geophys Geosyst 20: 260-276
Crosta X et al. (2022) Clim Past 18: 1729–1756
Fijn RC et al. (2012) Mar Ornithol 40: 81-87
Goto-Azuma K et al. (2019) Nat Commun 10: 3247
Hiller A et al. (1995) Radiocarbon 37: 171-180
McClymont EL et al. (2022) Clim Past 18: 381-403
Smith JA et al. (2010) Earth Planet Sci Lett 296: 287-298
Sprenk D et al. (2014) Clim Past 10: 1239-1251
Thatje S et al. (2008) Ecology 89: 682-692