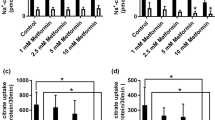
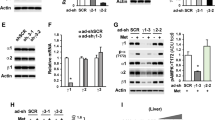
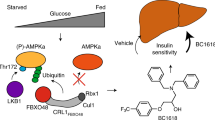
Abstract
Metformin is a first-line drug for the treatment of individuals with type 2 diabetes, yet its precise mechanism of action remains unclear. Metformin exerts its antihyperglycemic action primarily through lowering hepatic glucose production (HGP). This suppression is thought to be mediated through inhibition of mitochondrial respiratory complex I, and thus elevation of 5′-adenosine monophosphate (AMP) levels and the activation of AMP-activated protein kinase (AMPK), though this proposition has been challenged given results in mice lacking hepatic AMPK. Here we report that the AMP-inhibited enzyme fructose-1,6-bisphosphatase-1 (FBP1), a rate-controlling enzyme in gluconeogenesis, functions as a major contributor to the therapeutic action of metformin. We identified a point mutation in FBP1 that renders it insensitive to AMP while sparing regulation by fructose-2,6-bisphosphate (F-2,6-P2), and knock-in (KI) of this mutant in mice significantly reduces their response to metformin treatment. We observe this during a metformin tolerance test and in a metformin-euglycemic clamp that we have developed. The antihyperglycemic effect of metformin in high-fat diet–fed diabetic FBP1-KI mice was also significantly blunted compared to wild-type controls. Collectively, we show a new mechanism of action for metformin and provide further evidence that molecular targeting of FBP1 can have antihyperglycemic effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rena, G., Pearson, E. R. & Sakamoto, K. Molecular mechanism of action of metformin: old or new insights? Diabetologia 56, 1898–1906 (2013).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
El-Mir, M. Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000).
Bridges, H. R., Jones, A. J., Pollak, M. N. & Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 462, 475–487 (2014).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Vogt, J., Traynor, R. & Sapkota, G. P. The specificities of small molecule inhibitors of the TGFß and BMP pathways. Cell. Signal. 23, 1831–1842 (2011).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 (2010).
Miller, R. A. et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 (2013).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Hasenour, C. M. et al. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. J. Biol. Chem. 289, 5950–5959 (2014).
Vincent, M. F., Marangos, P. J., Gruber, H. E. & Van den Berghe, G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40, 1259–1266 (1991).
Pagliara, A. S., Karl, I. E., Keating, J. P., Brown, B. I. & Kipnis, D. M. Hepatic fructose-1,6-diphosphatase deficiency. A cause of lactic acidosis and hypoglycemia in infancy. J. Clin. Invest. 51, 2115–2123 (1972).
Bouskila, M. et al. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 12, 456–466 (2010).
Ouyang, J., Parakhia, R. A. & Ochs, R. S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 286, 1–11 (2011).
Gidh-Jain, M. et al. The allosteric site of human liver fructose-1,6-bisphosphatase. Analysis of six AMP site mutants based on the crystal structure. J. Biol. Chem. 269, 27732–27738 (1994).
Zhang, Y. et al. Fructose-1,6-bisphosphatase regulates glucose-stimulated insulin secretion of mouse pancreatic beta-cells. Endocrinology 151, 4688–4695 (2010).
Faupel, R. P., Seitz, H. J., Tarnowski, W., Thiemann, V. & Weiss, C. The problem of tissue sampling from experimental animals with respect to freezing technique, anoxia, stress and narcosis. A new method for sampling rat liver tissue and the physiological values of glycolytic intermediates and related compounds. Arch. Biochem. Biophys. 148, 509–522 (1972).
Erion, M. D. et al. MB06322 (CS-917): a potent and selective inhibitor of fructose 1,6-bisphosphatase for controlling gluconeogenesis in type 2diabetes. Proc. Natl. Acad. Sci. USA 102, 7970–7975 (2005).
Vincent, M. F., Erion, M. D., Gruber, H. E. & Van den Berghe, G. Hypoglycaemic effect of AICAriboside in mice. Diabetologia 39, 1148–1155 (1996).
Guigas, B. et al. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes 55, 865–874 (2006).
Vincent, M. F., Bontemps, F. & Van den Berghe, G. Substrate cycling between 5-amino-4-imidazolecarboxamide riboside and its monophosphate in isolated rat hepatocytes. Biochem. Pharmacol. 52, 999–1006 (1996).
Hunter, R. W. et al. Mechanism of action of compound-13: an α1-selective small molecule activator of AMPK. Chem. Biol. 21, 866–879 (2014).
Bailey, C. J., Wilcock, C. & Scarpello, J. H. Metformin and the intestine. Diabetologia 51, 1552–1553 (2008).
Yoshida, T. et al. Metformin primarily decreases plasma glucose not by gluconeogenesis suppression but by activating glucose utilization in a non-obese type 2 diabetes Goto-Kakizaki rats. Eur. J. Pharmacol. 623, 141–147 (2009).
Takashima, M. et al. Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes 59, 1608–1615 (2010).
Stepensky, D., Friedman, M., Raz, I. & Hoffman, A. Pharmacokinetic–pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metab. Dispos. 30, 861–868 (2002).
Duca, F. A. et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511 (2015).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
Gründemann, D., Gorboulev, V., Gambaryan, S., Veyhl, M. & Koepsell, H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372, 549–552 (1994).
Kjøbsted, R. et al. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 64, 2042–2055 (2015).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Patel, K. et al. The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat. Commun. 5, 4535 (2014).
Samuel, V. T. et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl. Acad. Sci. USA 106, 12121–12126 (2009).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Graham, G. G. et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50, 81–98 (2011).
Christensen, M. M. et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet. Genomics 21, 837–850 (2011).
Lalau, J. D., Lemaire-Hurtel, A. S. & Lacroix, C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin. Drug Investig. 31, 435–438 (2011).
Bleasby, K. et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica 36, 963–988 (2006).
Terada, T. et al. Molecular cloning, functional characterization and tissue distribution of rat H + /organic cation antiporter MATE1. Pharm. Res. 23, 1696–1701 (2006).
Gormsen, L. C. et al. In vivo imaging of human 11c-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 57, 1920–1926 (2016).
Jensen, J. B. et al. [11C]-labeled metformin distribution in the liver and small intestine using dynamic positron emission tomography in mice demonstrates tissue-specific transporter dependency. Diabetes 65, 1724–1730 (2016).
Chen, L. et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc. Natl. Acad. Sci. USA 111, 9983–9988 (2014).
Hawley, S. A., Gadalla, A. E., Olsen, G. S. & Hardie, D. G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51, 2420–2425 (2002).
Hawley, S. A. et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 (2010).
Argaud, D., Roth, H., Wiernsperger, N. & Leverve, X. M. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur. J Biochem. 213, 1341–1348 (1993).
McCarty, M. F. A proposal for the locus of metformin’s clinical action: potentiation of the activation of pyruvate kinase by fructose-1,6-diphosphate. Med. Hypotheses 52, 89–93 (1999).
van Poelje, P. D., Dang, Q. & Erion, M. D. Discovery of fructose-1,6-bisphosphatase inhibitors for the treatment of type 2 diabetes. Curr. Opin. Drug Discov. Devel. 10, 430–437 (2007).
Tao, H., Zhang, Y., Zeng, X., Shulman, G. I. & Jin, S. Niclosamide ethanolamine–induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20, 1263–1269 (2014).
Dang, Q. et al. Discovery of potent and specific fructose-1,6-bisphosphatase inhibitors and a series of orally-bioavailable phosphoramidase-sensitive prodrugs for the treatment of type 2 diabetes. J. Am. Chem. Soc. 129, 15491–15502 (2007).
Giroux, E., Williams, M. K. & Kantrowitz, E. R. Shared active sites of fructose-1,6-bisphosphatase. Arginine 243 mediates substrate binding and fructose 2,6-bisphosphate inhibition. J. Biol. Chem. 269, 31404–31409 (1994).
Tashima, Y., Mizunuma, H. & Hasegawa, M. Purification and properties of mouse liver fructose 1,6-bisphosphatase. J. Biochem. 86, 1089–1099 (1979).
Smiley, K. L. Jr., Berry, A. J. & Suelter, C. H. An improved purification, crystallization, and some properties of rabbit muscle 5′-adenylic acid deaminase. J. Biol. Chem. 242, 2502–2506 (1967).
Han, P., Han, G., McBay, H. & Johnson, J. Adenosine 5′-monophosphate-removing system in fructose-1,6-bisphosphatase assay mixture: a new approach. Anal. Biochem. 122, 269–273 (1982).
Nelson, S. W., Choe, J. Y., Honzatko, R. B. & Fromm, H. J. Mutations in the hinge of a dynamic loop broadly influence functional properties of fructose-1,6-bisphosphatase. J. Biol. Chem. 275, 29986–29992 (2000).
Chomczynski, P. & Rymaszewski, M. Alkaline polyethylene glycol-based method for direct PCR from bacteria, eukaryotic tissue samples, and whole blood. Biotechniques 40, 454 (2006). 456, 458.
Zarghi, A., Foroutan, S. M., Shafaati, A. & Khoddam, A. Rapid determination of metformin in human plasma using ion-pair HPLC. J. Pharm. Biomed. Anal. 31, 197–200 (2003).
Nakamura, K., Maeda, H. & Kawaguchi, H. Enzymatic assay of hemoglobin in tissue homogenates with chlorpromazine. Anal. Biochem. 165, 28–32 (1987).
Bosselaar, M., Smits, P., van Loon, L. J. & Tack, C. J. Intravenous AICAR during hyperinsulinemia induces systemic hemodynamic changes but has no local metabolic effect. J. Clin. Pharmacol. 51, 1449–1458 (2011).
Ayala, J.E. et al. Hyperinsulinemic–euglycemic clamps in conscious, unrestrained mice. J. Vis. Exp. (57), e3188 (2011).
Hasenour, C. M. et al. Mass spectrometry–based microassay of 2H and 13C plasma glucose labeling to quantify liver metabolic fluxes in vivo. Am. J. Physiol. Endocrinol. Metab. 309, E191–E203 (2015).
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Measuring deuterium enrichment of glucose hydrogen atoms by gas chromatography/mass spectrometry. Anal. Chem. 83, 3211–3216 (2011).
Young, J. D. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 30, 1333–1335 (2014).
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab. Eng. 8, 324–337 (2006).
Landau, B. R. et al. Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Invest. 98, 378–385 (1996).
Satapati, S. et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 53, 1080–1092 (2012).
Jakobsen, S. et al. A PET tracer for renal organic cation transporters, 11C-metformin: radiosynthesis and preclinical proof-of-concept studies. J. Nucl. Med. 57, 615–621 (2016).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408 (2001).
Keppler, D. & Decker, K. Glycogen determination with amyloglucosidase. in Methods of Enzymatic Analysis, Vol. 3 (ed. Bergmeyer, H.U.) 1127–1131 (Verlag Chemie, Weinheim, Germany, 1974).
Ryll, T. & Wagner, R. Improved ion-pair high-performance liquid chromatographic method for the quantification of a wide variety of nucleotides and sugar-nucleotides in animal cells. J. Chromatogr. 570, 77–88 (1991).
Noll, F. Methods of Enzymatic Analysis 3rd edn, Vol. 6 (ed. Bergmeyer, H. U.) 582–588 (Verlag Chemie, Weinheim, Germany, 1984).
Passonneau, J. V. & Lowry, O. H. Enzymatic analysis. A practical guide. (Humana Press, New York, 1993).
Racker, E. Methods of enzymatic analysis 1st edn (ed. Bergmeyer, H. U.) 160–163 (Verlag Chemie, Weinheim, Germany, 1965).
Van Schaftingen, E., Lederer, B., Bartrons, R. & Hers, H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur. J. Biochem. 129, 191–195 (1982).
Davidson, A. L. & Arion, W. J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch. Biochem. Biophys. 253, 156–167 (1987).
Castaño, J. G., Nieto, A. & Felíu, J. E. Inactivation of phosphofructokinase by glucagon in rat hepatocytes. J. Biol. Chem. 254, 5576–5579 (1979).
Blair, J. B., Cimbala, M. A., Foster, J. L. & Morgan, R. A. Hepatic pyruvate kinase. Regulation by glucagon, cyclic adenosine 3′-5′-monophosphate, and insulin in the perfused rat liver. J. Biol. Chem. 251, 3756–3762 (1976).
Petrescu, I. et al. Determination of phosphoenolpyruvate carboxykinase activity with deoxyguanosine 5′-diphosphate as nucleotide substrate. Anal. Biochem. 96, 279–281 (1979).
Saheki, S., Takeda, A. & Shimazu, T. Assay of inorganic phosphate in the mild pH range, suitable for measurement of glycogen phosphorylase activity. Anal. Biochem. 148, 277–281 (1985).
Srere, P. A. Citrate synthase. Methods Enzymol. 13, 3–11 (1969).
Thomas, J. A., Schlender, K. K. & Larner, J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal. Biochem. 25, 486–499 (1968).
Gilboe, D. P., Larson, K. L. & Nuttall, F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal. Biochem. 47, 20–27 (1972).
Stalmans, W. & Hers, H. G. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. A technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur. J. Biochem. 54, 341–350 (1975).
Acknowledgements
We thank M. Deak for molecular biology assistance and S. Jakobsen and J. Frøkiær for support in method development of the [11C]metformin-uptake study. We also thank E. Heikkilä for performing islet isolation, S. Ducommun for performing the pTBC1D1 blot, and S. Cotting for constructing Wollenberger tongs.GLUT2 antibody was provided by B. Thorens, GCK/HXK4 antibody was provided by M. Magnuson, GCKR antibody was provided by M. Shiota, G6PC antibody was provided by G. Mithieux, PFKFB1 antibody was provided by S. Baltrusch, pS33 PFKFB1 antibody was provided by J. Xie and pS8 GYS2 antibody was provided by J. Guinovart. This study was supported by Vanderbilt Mouse Metabolic Phenotyping Center Grant DK059637 (D.H.W.) and R37 DK050277 (D.H.W.), a Foundation Grant (FND 143277) from the Canadian Institutes of Health Research (F.S.), the Danish Council for Independent Research DFF—4183-00384 (N.J.) and the Novo Nordisk Foundation NNF13OC0003882 (N.J.). E.Z. was supported by a Sir Henry Wellcome postdoctoral fellowship. C.C.H. was supported by a Canadian Diabetes Association postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
R.W.H. and K.S. designed the study. R.W.H. performed all biochemical assays and the majority of in vivo experiments, assisted by K.S. Analysis of FBP1 structure and design of the mutants were performed by E.Z. and F.S. M.P. performed molecular cloning and mutagenesis of FBP1. N.J. and E.I.S. performed the [11C]metformin-uptake kinetics study and analyzed the data. C.C.H. and L.L. performed the metformin euglycemic clamp and analyzed the data. D.H.W. supervised C.C.H. and L.L. and contributed to interpretation of data from the clamp study. R.W.H and K.S. wrote the manuscript. All authors reviewed, edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.S. is a full-time employee of the Nestlé Institute of Health Sciences S.A., Switzerland.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figures and Tables
Supplementary Figures 1–9 and Supplementary Tables 1–7
Combined Source Data
Uncropped gels for Figs. 2h, 4k 5d, and Supplementary Fig. 6a
Rights and permissions
About this article
Cite this article
Hunter, R.W., Hughey, C.C., Lantier, L. et al. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat Med 24, 1395–1406 (2018). https://doi.org/10.1038/s41591-018-0159-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0159-7
This article is cited by
-
Chrysin-loaded PEGylated liposomes protect against alloxan-induced diabetic neuropathy in rats: the interplay between endoplasmic reticulum stress and autophagy
Biological Research (2024)
-
Metformin in gestational diabetes: physiological actions and clinical applications
Nature Reviews Endocrinology (2024)
-
TNFSF14+ natural killer cells prevent spontaneous abortion by restricting leucine-mediated decidual stromal cell senescence
The EMBO Journal (2024)
-
AMPK as a mediator of tissue preservation: time for a shift in dogma?
Nature Reviews Endocrinology (2024)
-
NF-kappa B signaling pathway is associated with metformin resistance in type 2 diabetes patients
Journal of Diabetes & Metabolic Disorders (2024)