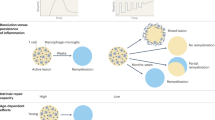
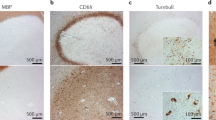
Abstract
Therapies for infiltrative inflammation in multiple sclerosis (MS) have advanced greatly, but neurodegeneration and compartmentalized inflammation remain virtually untargeted as in other diseases of the nervous system. Consequently, many therapies are available for the relapsing–remitting form of MS, but the progressive forms remain essentially untreated. The objective of the International Progressive MS Alliance is to expedite the development of effective therapies for progressive MS through new initiatives that foster innovative thinking and concrete advancements. Based on these principles, the Alliance is developing a new funding programme that will focus on experimental medicine trials. Here, we discuss the reasons behind the focus on experimental medicine trials, the strengths and weaknesses of these approaches and of the programme, and why we hope to advance therapies while improving the understanding of progression in MS. We are soliciting public and academic feedback, which will help shape the programme and future strategies of the Alliance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fox, R. J. et al. Setting a research agenda for progressive multiple sclerosis. The International Collaborative on Progressive MS. Mult. Scler. 18, 1534–1540 (2012).
Deshmukh, V. A. et al. A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332 (2013).
Mei, F. et al. Micropillar arrays as high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015).
Eleuteri, C. et al. A staged screening of registered drugs highlights remyelinating drug candidates for clinical trials. Sci. Rep. 7, 45780 (2017).
Rankin, K. A. et al. Selective estrogen receptor modulators enhance CNS remyelination independent of estrogen receptors. J. Neurosci. 39, 2184–2194 (2019).
Baker, D., Lidster, K., Sottomayor, A. & Amor, S. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biol. 12, e1001756 (2014).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altmann, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Baker, D. & Amor, S. Publication guidelines for refereeing and reporting on animal use in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 242, 78–83 (2012).
Baker, D. & Amor, S. Checklist for reporting and reviewing studies of experimental animal models of multiple sclerosis and related disorders. Mult. Scler. Relat. Disord. 1, 111–115 (2012).
Bergman, J. et al. Intrathecal treatment of rituximab in progressive MS. Neurology 91, 1893–1901 (2018).
Kosa, P. et al. Idebenone does not inhibit disability progression in primary progressive MS. Mult. Scler. Relat. Disord. https://doi.org/10.1016/j.msard.2020.102434 (2020).
Gnanapavan, S. et al. Biomarker report from the phase II lamotrigine trial in secondary progressive MS – neurofilament as a surrogate of disease progression. PLoS ONE 8, e70019 (2013).
Green, A. J. et al. Clemastine and fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomized, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017).
Komori, M. et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann. Clin. Transl. Neurol. 3, 166–179 (2016).
Brubaker, D. K. & Lauffenburger, D. A. Translating preclinical models to humans. Science 367, 742–743 (2020).
Brubaker, D. K. et al. An interspecies translation model implicates integrin signaling in infliximab-resistant inflammatory bowel disease. Sci. Signal. 13, eaay3258 (2020).
Kennedy, M. E. et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl Med. 8, 363ra150 (2016).
Egan, M. F. et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 380, 1408–1420 (2019).
Henley, D. et al. Preliminary results of a trial of atabecestat in pre-clinical Alzheimer’s disease. N. Engl. J. Med. 380, 1483–1485 (2019).
Li, S., Liu, L. & Selkoe, D. Verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 381, 388–389 (2019).
Dubuisson, N., Puentes, F., Giovannoni, G. & Gnanapavan, S. Science is 1% inspiration and 99% biomarkers. Mult. Scler. J. 23, 1442–1452 (2017).
Franklin, R. J. M. & Ffrench-Constant, C. Regenerating CNS myelin: from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Hauser, S. L. Progress in multiple sclerosis research: an example of bedside to bench. JAMA 324, 841–842 (2020).
FitzGerald, G. et al. The future of humans as model organisms. Science 361, 552–553 (2018).
Klotz, L. et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl Med. 11, eaao5563 (2019).
Insel, T. R. The NIMH experimental medicine initiative. World Psychiatry 15, 151–153 (2015).
Plenge, R. M. Disciplined approach to drug discovery and early development. Sci. Transl Med. 8, 349ps15 (2016).
Fang, H. et al. A genetics-led approach defines the drug target landscape of 30 immune-related traits. Nat. Genet. 51, 1082–1091 (2019).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377, 62–74 (2017).
Pardini, M., Cutter, G. & Sormani, M. P. Multiple sclerosis: clinical trial design 2019. Curr. Opin. Neurol. 32, 358–364 (2019).
Dickson, D. et al. The master observational trial: a new class of master protocol to advance precision medicine. Cell 180, 9–14 (2020).
Granovetter, M. The strength of weak ties. Am. J. Sociol. 78, 1360–1380 (1973).
Bodin, O. Collaborative environmental governance: Achieving collective action in social-ecological systems. Science 357, eaan1114 (2017).
Cipriani, A. & Barbui, C. What is a factorial trial. Epidemiol. Psychiatr. Sci. 22, 213–215 (2013).
Food and Drug Administration. FDA Guidance for Industry: codevelopment of two or more new investigational drugs for use in combination. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/codevelopment-two-or-more-new-investigational-drugs-use-combination (2013).
Romano, S. et al. Drug holiday of interferon beta 1b in multiple sclerosis: a pilot, randomized, single-blind study of non-inferiority. Front. Neurol. 10, 695 (2019).
Conway, D. & Cohen, J. A. Combination therapy in multiple sclerosis. Lancet Neurol. 9, 299–308 (2010).
Cohen, J. A. et al. Avonex combination trial in relapsing–remitting MS: rationale, design and baseline data. Mult. Scler. 14, 370–382 (2008).
Cohen, J. A. et al. Results of the Avonex Combination Trial (ACT) in relapsing-remitting MS. Neurology 72, 535–541 (2009).
Lublin, F. D. et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann. Neurol. 73, 327–340 (2013).
Mandolesi, G. et al. miR-142-3p is a key regulator of IL-1beta-dependent synaptopathy in neuroinflammation. J. Neurosci. 37, 546–561 (2017).
Lago, S. G. et al. Drug discovery for psychiatric disorders using high-content single-cell screening of signaling network responses ex vivo. Sci. Adv. 5, eaau9093 (2019).
Hartmann, F. J. et al. Comprehensive immune monitoring of clinical trials to advance human immunotherapy. Cell Rep. 28, 819–831 (2019).
Inoue, H., Nagata, N., Kurokawa, H. & Yamanaka, S. iPS cells: a game changer for future medicine. EMBO J. 33, 409–417 (2014).
Ashton, N. J. et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 16, 265–284 (2020).
Dubal, D. B. & Pleasure, S. J. Neural-derived extracellular vesicles in clinical trials: message in a bottle. JAMA Neurol. 76, 402–404 (2019).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Floris, M., Olla, S., Schlessinger, D. & Cucca, F. Genetic-driven druggable target identification and validation. Trends Genet. 34, 558–570 (2018).
Khramtsova, E. A., Davis, L. K. & Stranger, B. E. The role of sex in the genomics of human complex traits. Nat. Rev. Genet. 20, 173–190 (2019).
Alroughani, R. et al. Is time to reach EDSS 6.0 faster in patients with late-inset vs young-onset multiple sclerosis? PLoS ONE 11, e0165846 (2016).
Cinar, B. P. & Yorgun, Y. G. What we learned from the history of multiple sclerosis measurement: expanded disability status scale. Noro Psikiyatr. Ars. 55 (Suppl. 1), S69–S75 (2018).
Smith, K. A. et al. Comorbid disease burden among MS patients 1968-2012: a Swedish register-based cohort study. Mult. Scler. J. https://doi.org/10.1177/1352458520910497 (2020).
Berry, D. A. The Brave New World of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol. Oncol. 9, 951–959 (2015).
McFarland, H. F. et al. Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann. Neurol. 32, 758–766 (1992).
Frank, J. A. et al. Serial contrast-enhanced magnetic resonance imaging in patients with early relapsing-remitting multiple sclerosis: implications for treatment trials. Ann. Neurol. 36, S86–S90 (1994).
Nauta, J. J., Thompson, A. J., Barkhof, F. & Miller, D. H. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis patients: statistical power of parallel-groups and crossover designs. J. Neurol. Sci. 122, 6–14 (1994).
Pallmann, P. et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. https://doi.org/10.1186/s12916-018-1017-7 (2018).
The Adaptive Platform Trial Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat. Rev. Drug Discov. 18, 797–807 (2019).
Chataway, J. et al. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 19, 214–225 (2020).
Renfro, L. A. & Sargent, D. J. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann. Oncol. 28, 34–43 (2017).
Zarin, D. A., Goodman, S. N. & Kimmelman, J. Harms from uninformative clinical trials. JAMA https://doi.org/10.1001/jama.2019.9892 (2019).
Rieckmann, P. et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: A combined perspective from the MS in the 21st Century Steering Group. Mult. Scler. Rel. Disord. 19, 153–160 (2018).
Levitan, B. et al. Assessing the financial value of patient engagement: a quantitative approach from CTTI’s patient groups and clinical trials project. Ther. Innov. Regul. Sci. 52, 220–229 (2018).
Salvetti, M. et al. Steps towards collective sustainability in biomedical research. Trends Mol. Med. 24, 429–432 (2018).
Solaro, C. et al. Box and block test, hand grip strength and nine-hole peg test: correlations between three upper limb objective measures in multiple sclerosis. Eur. J. Neurol. 27, 2523–2530 (2020).
Newsome, S. D. et al. Longitudinal assessment of hand function in individuals with multiple sclerosis. Mult. Scler. Relat. Disord. 32, 107–113 (2019).
Kapoor, R. et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 95, 436–444 (2020).
Williams, T., Zetterberg, H. & Chataway, J. Neurofilaments in multiple sclerosis. A systematic review. J. Neurol. https://doi.org/10.1007/s00415-020-09917-x (2020).
Amiri, H. et al. Urgent challenger in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI. Neuroimage Clin. 19, 466–475 (2020).
Leocani, L., Guerrieri, S. & Comi, G. Visual evoked potentials as a biomarker in multiple sclerosis and associated optic neuritis. J. Neuroophtalmol. 38, 350–357 (2018).
Cruz-Herranz, A. et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 86, 2303–2309 (2016).
International Progressive Multiple Sclerosis Alliance. Collaborative Network Awards. https://www.progressivemsalliance.org/research/collaborative-network-awards/ (2020).
Simon, N. & Simon, R. Adaptive enrichment designs for clinical trials. Biostatistics 14, 613–625 (2013).
Koch, M. W. et al. The promise of futility trials in neurological diseases. Nat. Rev. Neurol. 11, 300–305 (2015).
Sibbaid, B. & Roberts, C. Understanding controlled trials. Crossover trials. BMJ 316, 1719 (1998).
Acknowledgements
The authors thank Dr Robert J. Fox for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
F.D., S.K., L.M. and M.S. researched data for the article. F.D., A.D., R.H., C.L., S.K., L.L., O.C., B.S., M.P.S., J.C., T.C. and M.S. contributed to writing of the manuscript. All authors made substantial contributions to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks B. Wienstock-Guttman, H. Weiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
International Progressive MS Alliance: https://www.progressivemsalliance.org/
Immune Tolerance Network: https://www.immunetolerance.org
Sage Bionetworks: http://sagebionetworks.org/
The MULTI-ACT project: https://www.multiact.eu/
Supplementary information
Rights and permissions
About this article
Cite this article
Dangond, F., Donnelly, A., Hohlfeld, R. et al. Facing the urgency of therapies for progressive MS — a Progressive MS Alliance proposal. Nat Rev Neurol 17, 185–192 (2021). https://doi.org/10.1038/s41582-020-00446-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00446-9
This article is cited by
-
Hybrid nanostructures for neurodegenerative disease theranostics: the art in the combination of biomembrane and non-biomembrane nanostructures
Translational Neurodegeneration (2024)
-
Bruton tyrosine kinase inhibitors for multiple sclerosis
Nature Reviews Neurology (2023)
-
Neuroglial components of brain lesions may provide new therapeutic strategies for multiple sclerosis
Neurological Sciences (2023)
-
Antibody Therapies for Progressive Multiple Sclerosis and for Promoting Repair
Neurotherapeutics (2022)
-
How common is active inflammation in progressive multiple sclerosis?
Nature Reviews Neurology (2021)