Key Points
-
Accumulation of neurotoxic forms of amyloid-β (Aβ) and tau proteins is the pathological hallmark of Alzheimer disease (AD)
-
Excess deposition of Aβ results from an imbalance between its production and clearance; in both early-onset and late-onset forms of AD, Aβ clearance seems already impaired at the prodromal stage
-
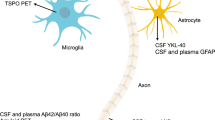
Aβ is removed from the brain by various overlapping and interacting clearance systems: degradation, blood–brain barrier (BBB) transport, interstitial fluid (ISF) bulk flow, and cerebrospinal fluid (CSF) absorption into the circulatory and peripheral lymphatic systems
-
Although most extracellular Aβ undergoes BBB clearance, the recently discovered glymphatic pathway seems to be important for Aβ clearance
-
Specific BBB transporters for tau have not been identified, suggesting that clearance of tau is less complex than that of Aβ, and mainly relies on degradation, ISF bulk flow, and CSF absorption
-
Precise understanding of the mechanisms of clearance dysfunction in AD is paramount to develop strategies to reduce excess deposition of neuroxic protein and to halt the related pathological changes
Abstract
Accumulation of toxic protein aggregates—amyloid-β (Aβ) plaques and hyperphosphorylated tau tangles—is the pathological hallmark of Alzheimer disease (AD). Aβ accumulation has been hypothesized to result from an imbalance between Aβ production and clearance; indeed, Aβ clearance seems to be impaired in both early and late forms of AD. To develop efficient strategies to slow down or halt AD, it is critical to understand how Aβ is cleared from the brain. Extracellular Aβ deposits can be removed from the brain by various clearance systems, most importantly, transport across the blood–brain barrier. Findings from the past few years suggest that astroglial-mediated interstitial fluid (ISF) bulk flow, known as the glymphatic system, might contribute to a larger portion of extracellular Aβ (eAβ) clearance than previously thought. The meningeal lymphatic vessels, discovered in 2015, might provide another clearance route. Because these clearance systems act together to drive eAβ from the brain, any alteration to their function could contribute to AD. An understanding of Aβ clearance might provide strategies to reduce excess Aβ deposits and delay, or even prevent, disease onset. In this Review, we describe the clearance systems of the brain as they relate to proteins implicated in AD pathology, with the main focus on Aβ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
29 March 2016
In the initially published version of this article, Figure 1 had an incomplete credit line. The correct credit line reads: Redrawn from Nedergaard, M. Garbage truck of the brain. Science 340, 1529-1530 (2013). Reprinted with permission from AAAS. This error has been corrected in the HTML and PDF versions of the article.
References
Reitz, C. & Mayeux, R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 88, 640–651 (2014).
Guerreiro, R. & Hardy, J. Genetics of Alzheimer's disease. Neurotherapeutics 11, 732–737 (2014).
Karch, C. M., Cruchaga, C. & Goate, A. M. Alzheimer's disease genetics: from the bench to the clinic. Neuron 83, 11–26 (2014).
Bertram, L. & Tanzi, R. E. The genetics of Alzheimer's disease. Prog. Mol. Biol. Transl. Sci. 107, 79–100 (2012).
Kim, D. H. et al. Genetic markers for diagnosis and pathogenesis of Alzheimer's disease. Gene 545, 185–193 (2014).
Bruni, A. C., Conidi, M. E. & Bernardi, L. Genetics in degenerative dementia: current status and applicability. Alzheimer Dis. Ass. Disord. 28, 199–205 (2014).
Selkoe, D. J. Aging, amyloid, and Alzheimer's disease: a perspective in honor of Carl Cotman. Neurochem. Res. 28, 1705–1713 (2003).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Zlokovic, B. V., Yamada, S., Holtzman, D., Ghiso, J. & Frangione, B. Clearance of amyloid β-peptide from brain: transport or metabolism? Nat. Med. 6, 718–719 (2000).
Potter, R. et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl. Med. 5, 189ra77 (2013).
Mawuenyega, K. G. et al. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science 330, 1774 (2010).
Reiman, E. M. et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case–control study. Lancet Neurol. 11, 1048–1056 (2012).
Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 9, 208–245 (2013).
Zlokovic, B. V. & Frangione, B. Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications. Madame Curie Bioscience Database [online], (2003).
Shibata, M. et al. Clearance of Alzheimer's amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J. Clin. Invest. 106, 1489–1499 (2000).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Louveau, A.E. et al. Structural and functional features of central nervous system lymphatic vessels. Nature http://dx.doi.org/10.1038/nature14432.
Wingo, T. S., Lah, J. J., Levey, A. I. & Cutler, D. J. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 69, 59–64 (2012).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 280–292 (2011).
Bishop, N. A., Lu, T. & Yankner, B. A. Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 (2010).
Hebert, L. E., Scherr, P. A., Bienias, J. L., Bennett, D. A. & Evans, D. A. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 60, 1119–1122 (2003).
Hetzel, L. 65 Years and Over Population: 2000. Census 2000 Brief (DIANE Publishing, 2008).
Alzheimer's Association. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 8, 131–168 (2012).
Potter, H. & Wisniewski, T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int. J. Alzheimers Dis. 2012, 489428 (2012).
Raber, J., Huang, Y. & Ashford, J. W. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging 25, 641–650 (2004).
Boutajangout, A. & Wisniewski, T. The innate immune system in Alzheimer's disease. Int. J. Cell Biol. 2013 (2013).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41, 1088–1093 (2009).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat. Genet. 45, 1452–1458 (2013).
Di Marco, L. Y. et al. Modifiable lifestyle factors in dementia: a systematic review of longitudinal observational cohort studies. J. Alzheimers Dis. (2014).
Reitz, C., Brayne, C. & Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 7, 137–152 (2011).
Barnes, D. E. & Yaffe, K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 10, 819–828 (2011).
Picchioni, D., Reith, R. M., Nadel, J. L. & Smith, C. B. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci. 4, 150–201 (2014).
Ju, Y.-E., Lucey, B. P. & Holtzman, D. M. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat. Rev. Neurol. 10, 115–119 (2014).
Spira, A. P. et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543 (2013).
Jack, C. R. Jr et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
de Leon, M. J., Bobinski, M., Convit, A. & De Santi, S. in Neurobiology of Mental Illness 1st edn Ch. 5 (eds Charney, D. S. & Nestler E. J.) 698–714 (Oxford University Press, 1999).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron 82, 756–771 (2014).
Thal, D. R. et al. Pathology of clinical and preclinical Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. 263 (Suppl. 2), S137–S145 (2013).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 (1990).
Blennow, K., Bogdanovic, N., Alafuzoff, I., Ekman, R. & Davidsson, P. Synaptic pathology in Alzheimer's disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the APOE4 allele. J. Neural Transm. 103, 603–618 (1996).
Giacobini, E. & Gold, G. Alzheimer disease therapy—moving from amyloid-β to tau. Nat. Rev. Neurol. 9, 677–686 (2013).
Thal, D. R., Griffin, W. S., de Vos, R. A. & Ghebremedhin, E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 115, 599–609 (2008).
Weller, R. O., Subash, M., Preston, S. D., Mazanti, I. & Carare, R. O. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 18, 253–266 (2008).
Mosconi, L. et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 64, 1860–1867 (2005).
Ferris, S. H. et al. Positron emission tomography in the study of aging and senile dementia. Neurobiol. Aging 1, 127–131 (1981).
de Leon, M. J. in Alzheimer: 100 Years and Beyond Illness 1st edn (eds Jucker, M. et al.) 385–390 (Springer, 2006).
de Leon, M. J. et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am. J. Neuroradiol. 14, 897–906 (1993).
Frisoni, G. B., Fox, N. C., Jack, C. R. Jr, Scheltens, P. & Thompson, P. M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 6, 67–77 (2010).
Cohen, A. D. & Klunk, W. E. Early detection of Alzheimer's disease using PiB and FDG PET. Neurobiol. Disease (2014).
Blennow, K. CSF biomarkers for AD: state of the art and new developments. Neurobiol. Aging 35, S3–S3 (2014).
Harada, R. et al. [18F]THK-5117 PET for assessing neurofibrillary pathology in Alzheimer's disease Eur. J. Nucl. Med. Mol. Imaging 42, 1052–1061 (2015).
Balasubramanian, A. B., Kawas, C. H., Peltz, C. B., Brookmeyer, R. & Corrada, M. M. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology 79, 915–921 (2012).
Blennow, K., Hampel, H., Weiner, M. & Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 (2010).
Villemagne, V. L., Fodero-Tavoletti, M. T., Masters, C. L. & Rowe, C. C. Tau imaging: early progress and future directions. Lancet Neurol. 14, 114–124 (2015).
Kiffin, R., Bandyopadhyay, U. & Cuervo, A. M. Oxidative stress and autophagy. Antioxid. Redox Signal. 8, 152–162 (2006).
Yin, K.-J. et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. J. Neurosci. 26, 10939–10948 (2006).
Wilcock, D. M. et al. Microglial activation facilitates Aβ plaque removal following intracranial anti-Aβ antibody administration. Neurobiol. Dis. 15, 11–20 (2004).
Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 106, 1261–1266 (2009).
Guenette, S. Y. Astrocytes: a cellular player in Aβ clearance and degradation. Trends Mol. Med. 9, 279–280 (2003).
Abbott, N. J., Patabendige, A. A., Dolman, D. E., Yusof, S. R. & Begley, D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 37, 13–25 (2010).
Zlokovic, B. V., Begley, D. J. & Chain-Eliash, D. G. Blood–brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine). Brain Res. 336, 125–132 (1985).
Zlokovic´, B. V., Lipovac, M. N., Begley, D. J., Davson, H. & Rakic´, L. Transport of leucine-enkephalin across the blood–brain barrier in the perfused guinea pig brain. J. Neurochem. 49, 310–315 (1987).
Zlokovic, B. V. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm. Res. 12, 1395–1406 (1995).
Hermann, D. M. & ElAli, A. The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci. Signal. 5, re4 (2012).
Thrane, A. S., Thrane, V. R. & Nedergaard, M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 37, 620–628 (2014).
Syková, E. & Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 88, 1277–1340 (2008).
Wong, A. D. et al. The blood–brain barrier: an engineering perspective. Front. Neuroeng. 6, 7 (2013).
Felgenhauer, K. Protein filtration and secretion at human body fluid barriers. Pflügers Arch. 384, 9–17 (1980).
Mathiisen, T. M., Lehre, K. P., Danbolt, N. C. & Ottersen, O. P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103 (2010).
Garai, K., Crick, S. L., Mustafi, S. M. & Frieden, C. Expression and purification of amyloid-β peptides from Escherichia coli. Protein Expr. Purif. 66, 107–112 (2009).
Yang, L. et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 11, 107 (2013).
Abbott, N. J. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 45, 545–552 (2004).
Milford, H. in Studies in Intracranial Physiology and Surgery 1st edn Ch. 1 (ed. Cushing, H.) 1–50 (Oxford University Press, 1926).
Loukas, M. et al. The lymphatic system: a historical perspective. Clin. Anat. 24, 807–816 (2011).
Fenstermacher, J. & Patlak, C. in Fluid Environment of the Brain 1st edn Ch. 12 (ed. Cserr, H.) 201–214 (Academic Press, 1975).
Cserr, H. F. Physiology of the choroid plexus. Physiol. Rev. 51, 273–311 (1971).
Cserr, H., Cooper, D., Suri, P. & Patlak, C. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 240, F319–F328 (1981).
Rennels, M. L., Gregory, T. F., Blaumanis, O. R., Fujimoto, K. & Grady, P. A. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326, 47–63 (1985).
Pullen, R. G., DePasquale, M. & Cserr, H. F. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am. J. Physiol. 253, F538–F545 (1987).
Szentistvanyi, I., Patlak, C. S., Ellis, R. A. & Cserr, H. F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246, F835–F844 (1984).
Carare, R. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 34, 131–144 (2008).
Preston, S., Steart, P., Wilkinson, A., Nicoll, J. & Weller, R. Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid β from the human brain. Neuropathol. Appl. Neurobiol. 29, 106–117 (2003).
Hawkes, C. A., Jayakody, N., Johnston, D. A., Bechmann, I. & Carare, R. O. Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy. Brain Pathol. 24, 396–403 (2014).
Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 (2013).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 (2014).
Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: a beginner's guide. Neurochem. Res. http://dx.doi.org/10.1007/s11064-015-1581-6.
Arbel-Ornath, M. et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer's disease mouse models. Acta Neuropathol. 126, 353–364 (2013).
Nimmerjahn, A. Two-photon imaging of microglia in the mouse cortex in vivo. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot069294.
Navari, R., Wei, E., Kontos, H. & Patterson, J. Comparison of the open skull and cranial window preparations in the study of the cerebral microcirculation. Microvasc. Res. 16, 304–315 (1978).
Bacskai, B. J. et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 7, 369–372 (2001).
Kawamura, S. et al. An improved closed cranial window technique for investigation of blood–brain barrier function and cerebral vasomotor control in the rat. Int. J. Microcirc. Clin. Exp. 9, 369–383 (1990).
Igarashi, H., Tsujita, M., Kwee, I. L. & Nakada, T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. Neuroreport 25, 39–43 (2014).
Oreškovic´, D. & Klarica, M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 64, 241–262 (2010).
Bering E. A. Jr. Water exchange of central nervous system and cerebrospinal fluid. J. Neurosurgery 9, 275–287 (1952).
Johanson, C. E. et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5, 10 (2008).
Wraith, D. C. & Nicholson, L. B. The adaptive immune system in diseases of the central nervous system. J. Clin. Invest. 122, 1172–1179 (2012).
Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 7, 9 (2010).
Bradbury, M., Cserr, H. & Westrop, R. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 240, F329–F336 (1981).
Bradbury, M. & Westrop, R. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol. 339, 519–534 (1983).
Bero, A. W. et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14, 750–756 (2011).
Neve, R. L. & McPhie, D. L. Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis. Biochim. Biophys. Acta 1772, 430–437 (2007).
Chow, V. W., Mattson, M. P., Wong, P. C. & Gleichmann, M. An overview of APP processing enzymes and products. Neuromol. Med. 12, 1–12 (2010).
Blennow, K., de Leon, M. J. & Zetterberg, H. Alzheimer's disease. Lancet 368, 387–403 (2006).
Zheng, L. et al. Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system. Transl. Neurodegener. 1, 19 (2012).
Morris, A. W., Carare, R. O., Schreiber, S. & Hawkes, C. A. The cerebrovascular basement membrane: role in the clearance of β-amyloid and cerebral amyloid angiopathy. Front. Aging Neurosci. 6, 251 (2014).
Mueller-Steiner, S. et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron 51, 703–714 (2006).
Moro, M. L. et al. APP mutations in the Aβ coding region are associated with abundant cerebral deposition of Aβ38. Acta Neuropathol. 124, 809–821 (2012).
Glabe, C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J. Mol. Neurosci. 17, 137–145 (2001).
Dawkins, E. & Small, D. H. Insights into the physiological function of the beta-amyloid precursor protein: beyond Alzheimer's disease. J. Neurochem. 129, 756–769 (2014).
Jarrett, J. T., Berger, E. P. & Lansbury, P. T. Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
Braak, H., Zetterberg, H., Del Tredici, K. & Blennow, K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 126, 631–641 (2013).
Grimm, M. O. et al. Neprilysin and Aβ clearance: impact of the APP intracellular domain in NEP regulation and implications in Alzheimer's disease. Front. Aging Neurosci. 5, 98 (2013).
Lührs, T. et al. 3D structure of Alzheimer's amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. USA 102, 17342–17347 (2005).
Schnabel, J. Amyloid: little proteins, big clues. Nature 475, S12–S14 (2011).
Perez, F. P. et al. Late-onset Alzheimer's disease, heating up and foxed by several proteins: pathomolecular effects of the aging process. J. Alzheimers Dis. 40, 1–17 (2014).
Zhang, L., Sheng, R. & Qin, Z. The lysosome and neurodegenerative diseases. Acta Biochim. Biophys. Sin. (Shanghai) 41, 437–445 (2009).
Querfurth, H. W. & LaFerla, F. M. Mechanisms of disease. N. Engl. J. Med. 362, 329–344 (2010).
Iwata, N. et al. Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552 (2001).
Miners, J. S. et al. Aβ-degrading enzymes in Alzheimer's disease. Brain Pathol. 18, 240–252 (2008).
Nalivaeva, N. N., Beckett, C., Belyaev, N. D. & Turner, A. J. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer's disease? J. Neurochem. 120, 167–185 (2012).
Eckman, E. A., Reed, D. K. & Eckman, C. B. Degradation of the Alzheimer's amyloid β peptide by endothelin-converting enzyme. J. Biol. Chem. 276, 24540–24548 (2001).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Qiu, W. Q. et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 273, 32730–32738 (1998).
Yoon, S. S. & Jo, S. A. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer's disease. Biomol. Ther. (Seoul) 20, 245–255 (2012).
Wang, D.-S., Iwata, N., Hama, E., Saido, T. C. & Dickson, D. W. Oxidized neprilysin in aging and Alzheimer's disease brains. Biochem. Biophys. Res. Commun. 310, 236–241 (2003).
Yasojima, K., McGeer, E. & McGeer, P. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 919, 115–121 (2001).
Lim, N. K. et al. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer's disease. J. Alzheimers Dis. 26, 779–786 (2011).
Morrone, C. D., Liu, M., Black, S. E. & McLaurin, J. Interaction between therapeutic interventions for Alzheimer's disease and physiological Aβ clearance mechanisms. Front. Aging Neurosci. 7 (2015).
Lucin, K. M. & Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122 (2009).
Cho, M. H. et al. Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10, 1761–1775 (2014).
Nagele, R. G., D'Andrea, M. R., Lee, H., Venkataraman, V. & Wang, H.-Y. Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 971, 197–209 (2003).
Lee, C. D. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 (2010).
Nixon, R. A. & Cataldo, A. M. Lysosomal system pathways: genes to neurodegeneration in Alzheimer's disease. J. Alzheimers Dis. 9, 277–289 (2006).
Chesser, A. S., Pritchard, S. M. & Johnson, G. V. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front. Neurol. 4, 122 (2013).
Pascale, C. L. et al. Amyloid-beta transporter expression at the blood–CSF barrier is age-dependent. Fluids Barriers CNS 8, 21 (2011).
Ito, S., Ohtsuki, S. & Terasaki, T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-β peptide (1–40) across the rat blood–brain barrier. Neurosci. Res. 56, 246–252 (2006).
Ito, S., Ohtsuki, S., Kamiie, J., Nezu, Y. & Terasaki, T. Cerebral clearance of human amyloid-β peptide (1–40) across the blood–brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 103, 2482–2490 (2007).
Ito, S., Ueno, T., Ohtsuki, S. & Terasaki, T. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-β peptide(1–40) in mouse: involvement of an LRP-1-independent pathway. J. Neurochem. 113, 1356–1363 (2010).
Panzenboeck, U. et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood–brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 277, 42781–42789 (2002).
Akanuma, S. et al. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-β peptide (1–40) at the blood–brain barrier. Neurochem. Int. 52, 956–961 (2008).
Fitz, N. F. et al. Abca1 deficiency affects Alzheimer's disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J. Neurosci. 32, 13125–13136 (2012).
ElAli, A. & Rivest, S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer's disease. Front. Physiol. 4, 45 (2013).
Ito, S. et al. Involvement of insulin-degrading enzyme in insulin- and atrial natriuretic peptide-sensitive internalization of amyloid-beta peptide in mouse brain capillary endothelial cells. J. Alzheimers Dis. 38, 185–200 (2014).
Deane, R. et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122, 1377 (2012).
Zlokovic, B. V., Deane, R., Sagare, A. P., Bell, R. D. & Winkler, E. A. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid beta-peptide elimination from the brain. J. Neurochem. 115, 1077–1089 (2010).
Pahnke, J., Langer, O. & Krohn, M. Alzheimer's and ABC transporters—new opportunities for diagnostics and treatment. Neurobiol. Dis. 72, A54–A60. (2014).
Cai, Z. Y., Yan, L. J. & Ratka, A. Telomere shortening and Alzheimer's disease. Neuromol. Med. 15, 25–48 (2013).
Krstic, D. & Knuesel, I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 9, 25–34 (2013).
Kanekiyo, T., Xu, H. & Bu, G. ApoE and Aβ in Alzheimer's disease: accidental encounters or partners? Neuron 81, 740–754 (2014).
Verghese, P. B. et al. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl Acad. Sci. USA 110, E1807–E1816 (2013).
Liu, C.-C., Kanekiyo, T., Xu, H. & Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118 (2013).
Wildsmith, K. R., Holley, M., Savage, J. C., Skerrett, R. & Landreth, G. E. Evidence for impaired amyloid beta clearance in Alzheimer's disease. Alzheimers Res. Ther. 5, 33 (2013).
Miyata, M. & Smith, J. D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 14, 55–61 (1996).
Smith, M. A., Rottkamp, C. A., Nunomura, A., Raina, A. K. & Perry, G. Oxidative stress in Alzheimer's disease. Biochim. Biophys. Acta 1502, 139–144 (2000).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Li, M., Chen, L., Lee, D. H., Yu, L.-C. & Zhang, Y. The role of intracellular amyloid β in Alzheimer's disease. Prog. Neurobiol. 83, 131–139 (2007).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Sagare, A. P., Bell, R. D. & Zlokovic, B. V. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer's disease. J. Alzheimers Dis. 33, S87–S100 (2013).
Weller, R. O. et al. Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am. J. Pathol. 153, 725–733 (1998).
Hawkes, C. A. et al. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS ONE 7, e41636 (2012).
Carare, R. O. et al. Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer's disease. Acta Neuropathol. Commun. 1, 48 (2013).
Hawkes, C. A. et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443 (2011).
Weller, R. O., Hawkes, C. A., Carare, R. O. & Hardy, J. Does the difference between PART and Alzheimer's disease lie in the age-related changes in cerebral arteries that trigger the accumulation of Aβ and propagation of tau? Acta Neuropathol. 129, 763–766 (2015).
Pezzini, A. & Padovani, A. Cerebral amyloid angiopathy-related hemorrhages. Neurol. Sci. 29, 260–263 (2008).
Sakai, K. et al. Aβ immunotherapy for Alzheimer's disease: effects on apoE and cerebral vasculopathy. Acta Neuropathol. 128, 777–789 (2014).
Schley, D., Carare-Nnadi, R., Please, C., Perry, V. & Weller, R. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 238, 962–974 (2006).
Hawkes, C. A. et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell 12, 224–236 (2013).
Hawkes, C. A., Gentleman, S. M., Nicoll, J. A. & Carare, R. O. Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring. J. Pathol. 235, 619–631 (2015).
Manousopoulou, A. et al. Are you also what your mother eats? Distinct proteomic portrait as a result of maternal high-fat diet in the cerebral cortex of the adult mouse. Int. J. Obes. (Lond.) (2015).
Iliff, J. J. & Nedergaard, M. Is there a cerebral lymphatic system? Stroke 44, S93–S95 (2013).
Stoodley, M. A., Brown, S. A., Brown, C. J. & Jones, N. R. Arterial pulsation-dependent perivascular cerebrospinal fluid flow into the central canal in the sheep spinal cord. J. Neurosurg. 86, 686–693 (1997).
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Maurizi, C. Recirculation of cerebrospinal fluid through the tela choroidae is why high levels of melatonin can be found in the lateral ventricles. Med. Hypotheses 35, 154–158 (1991).
Nedergaard, M. Garbage truck of the brain. Science 340, 1529–1530 (2013).
Hartl, F. U. & Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581 (2009).
Yang, W. et al. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol. Cell. Neurosci. 49, 406–414 (2012).
Ren, Z. et al. 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 33, 834–845 (2013).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
McKinley, J., McCarthy, A. & Lynch, T. Don't lose sleep over neurodegeneration-it helps clear amyloid beta. Front. Neurol. 4, 206 (2013).
Mendelsohn, A. R. & Larrick, J. W. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 16, 518–523 (2013).
O'Donnell, J., Ding, F. & Nedergaard, M. Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr. Sleep Med. Rep. 1, 1–8 (2015).
Wu, Y.-H. & Swaab, D. F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med. 8, 623–636 (2007).
Ju, Y.-E. et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 70, 587–593 (2013).
Musiek, E. S. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front. Pharmacol. 6, 29 (2015).
Deane, R. et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43, 333–344 (2004).
Lin, T.-W. et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 118, 189–197 (2015).
Herring, A. et al. Environmental enrichment counteracts Alzheimer's neurovascular dysfunction in TgCRND8 mice. Brain Pathol. 18, 32–39 (2008).
Richter, H. et al. Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav. Brain Res. 190, 74–84 (2008).
Marques, F., Sousa, J. C., Sousa, N. & Palha, J. A. Blood–brain-barriers in aging and in Alzheimer's. Mol. Neurodegener. 8, 38 (2013).
Picken, M. M. The changing concepts of amyloid. Arch. Pathol. Lab. Med. 125, 38–43 (2001).
Nakada, T., Igarashi, H., Suzuki, Y. & Kwee, I. Alzheimer patients show significant disturbance in water influx into CSF space strongly supporting β-amyloid clearance hypothesis [abstract S58.0001]. Neurology 82 (Suppl. 1) S58.001 (2014).
Serot, J. M., Zmudka, J. & Jouanny, P. A possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer's disease. J. Alzheimers Dis. 30, 17–26 (2012).
Erickson, M. A. & Banks, W. A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J. Cereb. Blood Flow Metab. 33, 1500–1513 (2013).
Fujiyoshi, M. et al. Amyloid-β peptide(1–40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood–cerebrospinal fluid barrier. J. Neurochem. 118, 407–415 (2011).
Silverberg, G. D., Mayo, M., Saul, T., Rubenstein, E. & McGuire, D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2, 506–511 (2003).
Silverberg, G., Mayo, M., Saul, T., Fellmann, J. & McGuire, D. Elevated cerebrospinal fluid pressure in patients with Alzheimer's. Cerebrospinal Fluid Res. 3, 7 (2006).
Pappolla, M. et al. Evidence for lymphatic Aβ clearance in Alzheimer's transgenic mice. Neurobiol. Dis. 71, 215–219 (2014).
Shea, T. & Beermann, M. Respective roles of neurofilaments, microtubules, MAP1B, and tau in neurite outgrowth and stabilization. Mol. Biol. Cell 5, 863–875 (1994).
Binder, L. I., Guillozet-Bongaarts, A. L., Garcia-Sierra, F. & Berry, R. W. Tau, tangles, and Alzheimer's disease. Biochim. Biophys. Acta 1739, 216–223 (2005).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211, 387–393 (2014).
Avila, J., Simon, D., Diaz-Hernandez, M., Pintor, J. & Hernandez, F. Sources of extracellular tau and its signaling. J. Alzheimers Dis. 40, (Suppl. 1) S7–S15 (2014).
Irazuzta, J. E., de Courten-Myers, G., Zemlan, F. P., Bekkedal, M. Y. & Rossi, J. 3rd. Serum cleaved tau protein and neurobehavioral battery of tests as markers of brain injury in experimental bacterial meningitis. Brain Res. 913, 95–105 (2001).
Castillo-Carranza, D. L. et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci. 34, 4260–4272 (2014).
Litersky, J. M. & Johnson, G. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J. Biol. Chem. 267, 1563–1568 (1992).
Medina, M. & Avila, J. The role of extracellular tau in the spreading of neurofibrillary pathology. Front. Cell. Neurosci. 8, 13 (2014).
Gómez-Ramos, A. et al. Characteristics and consequences of muscarinic receptor activation by tau protein. Europ. Neuropsychopharmacol. 19, 708–717 (2009).
David, D. C. et al. Proteasomal degradation of tau protein. J. Neurochemist. 83, 176–185 (2002).
Cirrito, J. R. et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 23, 8844–8853 (2003).
Iliff, J. J. et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 (2014).
Plog, B. A. et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 35, 518–526 (2015).
Bateman, R. J. et al. Quantifying CNS protein production and clearance rates in humans using in vivo stable isotope labeling, immunoprecipitation, and tandem mass spectrometry. Nat. Med. 12, 856 (2006).
Acknowledgements
The authors acknowledge the following grants: NIH/NIA/NHLBI AG022374, AG13616, AG12101 and AG008051 (to M.J.d.L.), HL118624 (to R.S.O), HL111724 (to L.G.), AG20245 and AG008051 (to T.W.), and NIH/NINDS NS028642 (to C.N.). K.B. has received funding from the Torsten Söderberg Foundation at the Royal Swedish Academy of Sciences, and H.Z. has received funding from the Swedish Research Council and the Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Contributions
M.J.d.L., J.M.T.-C., R.O.C., R.S.O., T.B., H.R., C.N., B.V.Z., K.B., H.Z. and T.W. researched data for article. M.J.d.L., J.M.T.-C., R.O.C., B.V.Z., H.Z. and T.W. wrote the article. M.J.d.L. and J.M.T.-C. provided substantial contributions to discussion of the content. All authors participated in reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.B. and H.Z. are co-founders of Brain Biomarker Solutions. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Tarasoff-Conway, J., Carare, R., Osorio, R. et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol 11, 457–470 (2015). https://doi.org/10.1038/nrneurol.2015.119
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.119