Key Points
-
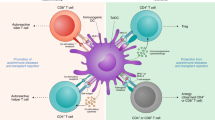
Tolerogenic dendritic cells (DCs) of various subsets have been described in rodents and humans. They offer potential as therapeutic tools to ameliorate or prevent transplant rejection or graft-versus-host disease (GVHD), or to treat autoimmune disorders.
-
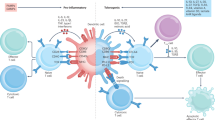
Tolerogenic DCs include immature, maturation-resistant or alternatively activated DCs that express surface MHC class I and class II molecules, have a low co-stimulatory to inhibitory signal ratio and have an impaired ability to synthesize T helper 1 (TH1)-cell-driving cytokines (such as interleukin-12p70). Various anti-inflammatory and immunosuppressive agents potentiate or confer tolerogenicity on DCs (in vitro or in vivo).
-
Growth-factor-induced DC expansion (mobilization) in donor or host tissues has resulted in variable transplant outcomes leading to tolerance or exacerbation of rejection, depending on the model.
-
Donor- or host-derived DCs, adoptively transferred to allograft recipients or targeted in situ (allopeptides, apoptotic cells or exosomes) can potentiate long-term transplant survival in normal hosts; this effect is potentiated by conventional and experimental immunosuppressive agents, including the co-stimulation blocking molecules.
-
Mechanisms by which DCs mediate their tolerogenic properties include T-cell deletion or anergy, polarization of TH2-cell responses and expansion or induction of regulatory T cells (with the ability to suppress T cells that recognize alloantigen through the direct or indirect pathways).
-
DC function may be modified in situ by local microenvironmental factors (for example, in the liver) such that they acquire tolerogenic properties.
-
There is a pressing need to ascertain whether the ability of rodent DCs to promote transplant tolerance can be extrapolated from rodents to non-human primates, which is likely to provide a better index of their potential for clinical application. Proof-of-principle studies show that autologous immature DCs can promote T-cell tolerance to model antigens in humans.
Abstract
In recent years, there has been a shift from the perception of dendritic cells (DCs) solely as inducers of immune reactivity to the view that these cells are crucial regulators of immunity, which includes their ability to induce and maintain tolerance. Advances in our understanding of the phenotypical and functional plasticity of DCs, and in our ability to manipulate their development and maturation in vitro and in vivo, has provided a basis for the therapeutic harnessing of their inherent tolerogenicity. In this Review, we integrate the available information on the role of DCs in the induction of tolerance, with a focus on transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007). References 2 and 3 are comprehensive and authoritative reviews of DC subsets and DC tolerogenicity.
Vremec, D., Pooley, J., Hochrein, H., Wu, L. & Shortman, K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164, 2978–2986 (2000).
Henri, S. et al. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167, 741–748 (2001).
Corcoran, L. et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170, 4926–4932 (2003).
Shortman, K. & Liu, Y. J. Mouse and human dendritic cell subtypes. Nature Rev. Immunol. 2, 151–161 (2002).
Asselin-Paturel, C. et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nature Immunol. 2, 1144–1150 (2001).
Nakano, H., Yanagita, M. & Gunn, M. D. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194, 1171–1178 (2001).
Bjorck, P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte–macrophage colony-stimulating factor-treated mice. Blood 98, 3520–3526 (2001).
Yrlid, U. & Macpherson, G. Phenotype and function of rat dendritic cell subsets. APMIS 111, 756–765 (2003).
Brocker, T., Riedinger, M. & Karjalainen, K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185, 541–550 (1997).
Menges, M. et al. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 195, 15–21 (2002).
Verginis, P., Li, H. S. & Carayanniotis, G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J. Immunol. 174, 7433–7439 (2005).
Hackstein, H. & Thomson, A. W. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nature Rev. Immunol. 4, 24–35 (2004). A detailed survey of how pharmacological agents regulate DC differentiation and maturation and promote their tolerogenic properties.
Morelli, A. E. & Thomson, A. W. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol. Rev. 196, 125–146 (2003).
Chauveau, C. et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 106, 1694–1702 (2005).
Hackstein, H. et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood 101, 4457–4463 (2003).
Turnquist, H. et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 178, 7018–7031 (2007).
Min, W. P. et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J. Immunol. 170, 1304–1312 (2003).
Penna, G. & Adorini, L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 164, 2405–2411 (2000).
Griffin, M. D. et al. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem. Biophys. Res. Commun. 270, 701–708 (2000).
Barrat, F. J. et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (TH1)- and TH2-inducing cytokines. J. Exp. Med. 195, 603–616 (2002).
Gregori, S. et al. Regulatory T cells induced by 1α, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 167, 1945–1953 (2001).
Liang, S. & Horuzsko, A. Mobilizing dendritic cells for tolerance by engagement of immune inhibitory receptors for HLA-G. Hum. Immunol. 64, 1025–1032 (2003).
Bonham, C. A. et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-κB oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J. Immunol. 169, 3382–3391 (2002).
Hill, J. A. et al. Immune modulation by silencing IL-12 production in dendritic cells using small interfering RNA. J. Immunol. 171, 691–696 (2003).
Maraskovsky, E. et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953–1962 (1996).
Pulendran, B. et al. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J. Exp. Med. 188, 2075–2082 (1998).
Coates, P. T. et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood 102, 2513–2521 (2003).
Eto, M., Hackstein, H., Kaneko, K., Nomoto, K. & Thomson, A. W. Promotion of skin graft tolerance across MHC barriers by mobilization of dendritic cells in donor hemopoietic cell infusions. J. Immunol. 169, 2390–2396 (2002).
Antonysamy, M. A. et al. Flt-3 ligand increases microchimerism but can prevent the therapeutic effect of donor bone marrow in transiently immunosuppressed cardiac allograft recipients. J. Immunol. 160, 4106–4113 (1998).
Hackstein, H. et al. Normal donor bone marrow is superior to Flt3 ligand-mobilized bone marrow in prolonging heart allograft survival when combined with anti-CD40L (CD154). Am. J. Transplant. 2, 609–617 (2002).
Coates, P. T. et al. In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation 77, 1080–1089 (2004).
Steptoe, R. J. et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J. Immunol. 159, 5483–5491 (1997).
Arpinati, M., Green, C. L., Heimfeld, S., Heuser, J. E. & Anasetti, C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95, 2484–2490 (2000).
Gould, D. S. & Auchincloss, H. Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol. Today 20, 77–82 (1999).
Herrera, O. B. et al. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 173, 4828–4837 (2004).
Thery, C. et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunol. 3, 1156–1162 (2002).
Harshyne, L. A., Watkins, S. C., Gambotto, A. & Barratt-Boyes, S. M. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J. Immunol. 166, 3717–3723 (2001).
Mueller, D. L., Jenkins, M. K. & Schwartz, R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol. 7, 445–480 (1989).
Lu, L., McCaslin, D., Starzl, T. E. & Thomson, A. W. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation 60, 1539–1545 (1995).
Fu, F. et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation 62, 659–665 (1996). The first account of the ability of immature myeloid DCs of donor origin to prolong organ allograft survival.
Lutz, M. B. et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur. J. Immunol. 30, 1813–1822 (2000). Describes how maturation-resistant DCs can induce indefinite organ allograft survival.
Cong, Y. et al. Generation of antigen-specific, Foxp3-expressing CD4+ regulatory T cells by inhibition of APC proteosome function. J. Immunol. 174, 2787–2795 (2005).
Wakkach, A. et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18, 605–617 (2003).
Yamazaki, S. et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198, 235–247 (2003).
Yamazaki, S. et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc. Natl Acad. Sci. USA 103, 2758–2763 (2006).
Tarbell, K. V., Yamazaki, S., Olson, K., Toy, P. & Steinman, R. M. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199, 1467–1477 (2004). Shows that antigen-specific T Reg cells can be expanded in vitro using DCs and that these T Reg cells suppress autoimmune disease.
Rastellini, C. et al. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 60, 1366–1370 (1995).
Lu, L. et al. Blockade of the CD40–CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation 64, 1808–1815 (1997).
Niimi, M. et al. Operational tolerance induced by pretreatment with donor dendritic cells under blockade of CD40 pathway. Transplantation 72, 1556–1562 (2001).
Hayamizu, K., Huie, P., Sibley, R. K. & Strober, S. Monocyte-derived dendritic cell precursors facilitate tolerance to heart allografts after total lymphoid irradiation. Transplantation 66, 1285–1291 (1998).
Selenko-Gebauer, N. et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J. Immunol. 170, 3637–3644 (2003).
Munn, D. H. et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297, 1867–1870 (2002).
Munn, D. H., Sharma, M. D. & Mellor, A. L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 172, 4100–4110 (2004).
Chang, C. C. et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature Immunol. 3, 237–243 (2002).
Steinbrink, K., Graulich, E., Kubsch, S., Knop, J. & Enk, A. H. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 99, 2468–2476 (2002).
Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192, 1213–1222 (2000).
Levings, M. K. et al. Differentiation of TR1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ TR cells. Blood 105, 1162–1169 (2005).
Suss, G. & Shortman, K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand- induced apoptosis. J. Exp. Med. 183, 1789–1796 (1996).
Belz, G. T. et al. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Grohmann, U. et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J. Immunol. 166, 277–283 (2001).
Grohmann, U. et al. IFN-γ inhibits presentation of a tumor/self peptide by CD8α− dendritic cells via potentiation of the CD8α+ subset. J. Immunol. 165, 1357–1363 (2000).
Fallarino, F. et al. Modulation of tryptophan catabolism by regulatory T cells. Nature Immunol. 4, 1206–1212 (2003). An elegant demonstration that ligation of CTLA4 by T Reg cells on DCs augments functional IDO expression.
Belladonna, M. L. et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 177, 130–137 (2006).
Grohmann, U. et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nature Immunol. 3, 1097–1101 (2002).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev. Immunol. 4, 762–774 (2004).
O'Connell, P. J. et al. Immature and mature CD8α+ dendritic cells prolong the survival of vascularized heart allografts. J. Immunol. 168, 143–154 (2002).
Siegal, F. P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Rissoan, M. C. et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283, 1183–1186 (1999).
Moseman, E. A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173, 4433–4442 (2004).
Gilliet, M. & Liu, Y. J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195, 695–704 (2002).
Ito, T. et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204, 105–115 (2007). References 74–76 show how pDCs can generate different types of regulatory T cells.
Fallarino, F. et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 173, 3748–3754 (2004).
Grohmann, U. et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nature Med. 13, 579–586 (2007).
Fugier-Vivier, I. J. et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J. Exp. Med. 201, 373–383 (2005).
Abe, M., Wang, Z., De Creus, A. & Thomson, A. W. Plasmacytoid dendritic cell precursors induce allogeneic T cell hyporesponsiveness and prolong heart graft survival. Am. J. Transplant. 5, 1808–1819 (2005).
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–2173 (1998).
Yu, G., Xu, X., Vu, M. D., Kilpatrick, E. D. & Li, X. C. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J. Exp. Med. 203, 1851–1858 (2006).
Garrovillo, M., Ali, A. & Oluwole, S. F. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific tolerance to rat cardiac allografts by allopeptide-pulsed host dendritic cells. Transplantation 68, 1827–1834 (1999). The first report that alloantigen-pulsed host DCs can be used to induce alloantigen-specific organ transplant tolerance.
Ali, A., Garrovillo, M., Jin, M. X., Hardy, M. A. & Oluwole, S. F. Major histocompatibility complex class I peptide-pulsed host dendritic cells induce antigen-specific acquired thymic tolerance to islet cells. Transplantation 69, 221–226 (2000).
Oluwole, O. O. et al. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulsed host myeloid and thymic dendritic cells. Diabetes 50, 1546–1552 (2001).
Taner, T., Hackstein, H., Wang, Z., Morelli, A. E. & Thomson, A. W. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 5, 228–236 (2005).
Peche, H., Trinite, B., Martinet, B. & Cuturi, M. C. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am. J. Transplant. 5, 255–267 (2005).
Beriou, G., Peche, H., Guillonneau, C., Merieau, E. & Cuturi, M. C. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation 79, 969–972 (2005).
Sato, K., Yamashita, N., Yamashita, N., Baba, M. & Matsuyama, T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity 18, 367–379 (2003).
Golshayan, D. et al. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood 109, 827–835 (2007). This study shows that DCs can expand alloantigen-specific T Reg cells in vitro that promote transplant tolerance.
Jiang, S., Camara, N., Lombardi, G. & Lechler, R. I. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood 102, 2180–2186 (2003).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001). A proof-of-principle study that shows that immature autologous DCs pulsed with nominal antigen can induce antigen-specific T-cell tolerance in healthy volunteers.
Ochando, J. C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature Immunol. 7, 652–662 (2006).
Baban, B. et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int. Immunol. 17, 909–919 (2005).
Mellor, A. L. et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 16, 1391–1401 (2004).
Mellor, A. L. et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J. Immunol. 175, 5601–5605 (2005).
Mirenda, V. et al. Modified dendritic cells coexpressing self and allogeneic major histocompatibility complex molecules: an efficient way to induce indirect pathway regulation. J. Am. Soc. Nephrol. 15, 987–997 (2004).
Garrod, K. R. et al. Targeted lymphoid homing of dendritic cells is required for prolongation of allograft survival. J. Immunol. 177, 863–868 (2006).
Matsue, H. et al. Immunosuppressive properties of CD95L-transduced 'killer' hybrids created by fusing donor- and recipient-derived dendritic cells. Blood 98, 3465–3472 (2001).
Morelli, A. E. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am. J. Transplant. 6, 254–261 (2006).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001). The first documentation that in situ targeting of DCs with monoclonal antibodies and antigen in the steady state is associated with antigen-specific T-cell tolerance.
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Larregina, A. T. & Morelli, A. E. in Handbook of Dendritic Dells: Biology, Diseases, and Therapies (eds Lutz, M. B., Romani, N. & Steinkasserer, A.) 591–618 (Wiley-VCH, Weinheim, 2006).
Morelli, A. E. et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101, 611–620 (2003).
Wang, Z. et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am. J. Transplant. 6, 1297–1311 (2006). The first report that in situ targeting of DCs with donor-derived apoptotic cells prolongs solid organ allograft survival.
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nature Rev. Immunol. 2, 569–579 (2002).
Morelli, A. E. et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266 (2004).
Peche, H. et al. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am. J. Transplant. 6, 1541–1550 (2006).
Qian, S., Thai, N. L., Lu, L., Fung, J. J. & Thomson, A. W. Liver transplant tolerance: mechanistic insights from animal models with particular reference to the mouse. Transplant. Rev. 11, 151–164 (1997).
Crispe, I. N. Hepatic T cells and liver tolerance. Nature Rev. Immunol. 3, 51–62 (2003).
Pillarisetty, V. G., Miller, G., Shah, A. B. & DeMatteo, R. P. GM-CSF expands dendritic cells and their progenitors in mouse liver. Hepatology 37, 641–652 (2003).
Sun, J., McCaughan, G. W., Gallagher, N. D., Sheil, A. G. & Bishop, G. A. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation 60, 233–236 (1995).
Shimizu, Y. et al. Restoration of tolerance to rat hepatic allografts by spleen-derived passenger leukocytes. Transpl. Int. 9, 593–595 (1996).
Lu, L. et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allgraft recipients in response to granulocyte/macrophage colony stimulating factor. J. Exp. Med. 182, 379–387 (1995). The novel finding that donor-derived DCs can be propagated from the bone marrow of animals that show spontaneous liver transplant tolerance but not from those that acutely reject heart allografts.
Demetris, A. et al. Analysis of chronic rejection and obliterative arteriolopathy. Possible contributions of donor antigen presenting cells and lymphatic disruption. Am. J. Pathol. 150, 563 (1997).
Qian, S. et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J. Immunol. 158, 4654–4661 (1997).
Sharland, A. et al. Evidence that apoptosis of activated T cells occurs in spontaneous tolerance of liver allografts and is blocked by manipulations which break tolerance. Transplantation 68, 1736–1745 (1999).
Li, W. et al. IL-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J. Immunol. 166, 5619–5628 (2001).
Randow, F. et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor β. J. Exp. Med. 181, 1887–1892 (1995).
van der Poll, T., Coyle, S. M., Moldawer, L. L. & Lowry, S. F. Changes in endotoxin-induced cytokine production by whole blood after in vivo exposure of normal humans to endotoxin. J. Infect. Dis. 174, 1356–1360 (1996).
Dobrovolskaia, M. A. & Vogel, S. N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 4, 903–914 (2002).
Kobayashi, K. et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202 (2002).
Medvedev, A. E., Lentschat, A., Wahl, L. M., Golenbock, D. T. & Vogel, S. N. Dysregulation of LPS-induced Toll-like receptor 4–MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169, 5209–5216 (2002).
Nakagawa, R. et al. SOCS-1 participates in negative regulation of LPS responses. Immunity 17, 677–687 (2002).
Ziegler-Heitbrock, H. W. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45, 13–26 (1995).
Wysocka, M. et al. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166, 7504–7513 (2001).
Tinsley, K. W. et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 171, 909–914 (2003).
De Creus, A. et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 174, 2037–2045 (2005).
Abe, M., Tokita, D., Raimondi, G. & Thomson, A. W. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct TH1-type responses. Eur. J. Immunol. 36, 2483–2493 (2006).
Ishiyama, K. et al. Induction of endotoxin tolerance inhibits alloimmune responses. Transpl. Immunol. 16, 158–165 (2006).
Liew, F. Y., Xu, D., Brint, E. K. & O'Neill L, A. Negative regulation of Toll-like receptor-mediated immune responses. Nature Rev. Immunol. 5, 446–458 (2005).
Acknowledgements
Owing to space limitations, many important studies could not be included, and we apologize for any such oversight. The work in our laboratories was supported in part by grants from the National Institutes of Health and the 2006 American Society of Transplantation Basic Science Career Development Award to A.E.M. We thank the members of our laboratories and colleagues in the field for their helpful suggestions and M. Freeman for administrative support.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Promotion of indefinite allograft survival by donor DC administration (PDF 177 kb)
Supplementary information S2 (table)
Promotion of indefinite allograft survival by bone–marrow–derived recipient DC administration (PDF 138 kb)
Related links
Glossary
- Conventional DC
-
A dendritic cell (DC) that already displays the morphology and function of a DC under steady-state conditions.
- Precursor DC
-
(Pre-DC). An immediate predecessor of a dendritic cell (DC) that, in the steady state, does not have the appearance or function of a DC (for example, circulating monocytes are pre-DCs of myeloid DCs and pre-plasmacytoid DCs (pre-pDCs) are pre-DCs of pDCs). Pre-DCs acquire the morphology and T-cell stimulatory ability of DCs with little or no cell division in the presence of microbial or inflammatory stimuli.
- Danger signal
-
An agent that, through the induction of pro-inflammatory mediators, initiates innate and adaptive immune responses. Exogenous danger signals (such as lipopolysaccharide) are derived from microbial pathogens. Endogenous danger signals (such as high-mobility group box 1 protein and heat shock proteins) are released by stressed or damaged cells.
- Graft-versus-host disease
-
(GVHD). A disease that results from the immunological attack by donor allogeneic T cells that are transferred along with the allograft (such as bone marrow, liver or gut allografts) of target recipient organs or tissues (such as the skin or gut). GVHD occurs in graft recipients that are unable to eliminate the host-reactive donor T cells owing to immunosuppression, immunological immaturity or tolerance of the recipient.
- T-cell anergy
-
A state of T-cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
- Alternatively activated DC
-
A dendritic cell (DC) that is activated after pretreatment with corticosteroids or anti-inflammatory cytokines, resulting in a stable, semi-mature DC that can induce T-cell hyporesponsiveness in vitro and prolong allograft survival or inhibit GVHD.
- FOXP3+CD4+CD25+ TReg cell
-
A naturally occurring regulatory T (TReg) cell that arises in the thymus, but is also detected in the periphery, expresses high levels of the transcription factor FOXP3 in the nucleus and inhibits autologous T-cell proliferative responses by a contact-dependent mechanism.
- RNA interference
-
A phenomenon in which the expression of a gene is inhibited when a double-stranded complementary RNA is introduced into the organism.
- FMS-like tyrosine kinase 3 ligand
-
(FLT3L). An endogenous cytokine that stimulates the proliferation of stem and progenitor cells through binding to the FLT3 receptor (a type III receptor tyrosine kinase member of the PDGF family). FLT3L administration substantially increases the number of DCs in lymphoid and non-lymphoid tissues.
- Chimerism
-
The presence of donor-derived cells (normally of haematopoietic origin) in the tissues of allograft recipients.
- Chronic rejection
-
Late graft rejection that is associated with tissue injury, mediated by chronic inflammation, alloantibodies and vascular pathology, which is believed to be caused by T- and B-cell-mediated immunity.
- 'Two-signal' hypothesis
-
The concept that both the MHC–peptide complex (signal 1) and co-stimulatory signals delivered by B7 family molecules expressed by APCs (signal 2) are required for T-cell activation. The absence of signal 2 results in the induction of T-cell anergy or deletion.
- T regulatory type 1 (TR1) cells
-
A subset of CD4+ regulatory T cells that secrete high levels of IL-10 and that downregulate TH1- and TH2-cell responses in vitro and in vivo by a contact-independent mechanism(s) mediated by the secretion of soluble IL-10 and TGFβ1.
- Third-party transplant
-
A graft from a non-identical strain (usually MHC-mismatched) that is used to assess the specificity of unresponsiveness to donor alloantigen in vivo.
- Graft-vesus-leukaemia (GVL) effect
-
The anti-tumour activity of donor T cells against residual leukaemic cells of the graft recipient following (allogeneic) bone marrow transplantation.
- Linked suppression
-
A mechanism of inhibition of the T-cell alloresponse by which an 'A' recipient that is rendered tolerant to a 'B' graft, accepts grafts from a B × C donor, but rejects tissues from an A × C donor.
- Delayed-type hypersensitivity
-
A cellular immune response to antigen that develops over 24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of TH1-cell-specific cytokines.
- Exosome
-
A membrane nanovesicle (<100 nm) that is produced by different cell types and released into the extracellular space by fusion of multivesicular bodies with the plasma membrane.
- Endotoxin tolerance or cross tolerance
-
A transient state of hyporesponsiveness of the host or of cultured macrophages and/or monocytes to LPS (endotoxin tolerance) or other TLR ligands (cross tolerance) following previous exposure to LPS.
Rights and permissions
About this article
Cite this article
Morelli, A., Thomson, A. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 7, 610–621 (2007). https://doi.org/10.1038/nri2132
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2132