Key Points
-
Siglecs are sialic-acid-binding immunoglobulin-like lectins that are mostly expressed by cells of the immune system.
-
Siglecs can be divided into two subsets based on their sequence similarity and evolutionary relatedness. The main subset comprises the CD33-related subgroup, which appears to be evolving rapidly, and shows important differences in repertoire between mammalian species, particularly between humans and the closely related great apes.
-
Many Siglecs have tyrosine-based signalling motifs, especially immunoreceptor tyrosine-based inhibitory motifs (ITIMs), that are implicated in cell signalling and endocytosis.
-
Low-affinity sialylated Siglec ligands are expressed abundantly on cells, which commonly results in the masking of the sialic-acid-binding site of Siglecs. Masking can be overcome by high-density and/or high-affinity ligands presented on opposing cells.
-
The Siglec CD22 is a well-characterized inhibitory receptor of B cells and has up to four ITIMs. Its cis interactions with α2–6-sialylated ligands are important for regulating signalling functions.
-
Sialoadhesin is a macrophage-restricted Siglec with 17 immunoglobulin domains. Its unusual length and the absence of signalling motifs suggest a predominant role in cell–cell interactions. This is also indicated in initial studies of sialoadhesin-deficient mice, which show impairment in development of experimentally induced autoimmune diseases.
-
The CD33-related Siglecs are expressed mostly in the innate immune system and appear to be important for regulating cellular expansion and activation. Most have two conserved tyrosine motifs, one of which is an ITIM important for recruitment of the SRC homology 2 (SH2)-domain-containing protein tyrosine phosphatase 1 (SHP1) and SHP2 tyrosine phosphatases and the suppressor of cytokine signalling 3 (SOCS3).
-
Many human pathogens can express sialic acids that are recognized by several Siglecs. It is possible that Siglec-dependent recognition of these pathogen glycans leads to altered immune responses, either to the advantage or to the detriment of the pathogen.
-
Some CD33-related Siglecs of the immune system, such as Siglec-H and Siglec-14, lack ITIMs. Instead, they associate with the immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor DAP12 that typically triggers cell activation. Siglec-14 is highly related to the ITIM-bearing Siglec-5 and they can therefore be considered as 'paired receptors'.
Abstract
Cell surfaces in the immune system are richly equipped with a complex mixture of glycans, which can be recognized by diverse glycan-binding proteins. The Siglecs are a family of sialic-acid-binding immunoglobulin-like lectins that are thought to promote cell–cell interactions and regulate the functions of cells in the innate and adaptive immune systems through glycan recognition. In this Review, we describe recent studies on signalling mechanisms and discuss the potential role of Siglecs in triggering endocytosis and in pathogen recognition. Finally, we discuss the postulated functions of the recently discovered CD33-related Siglecs and consider the factors that seem to be driving their rapid evolution.
Similar content being viewed by others
Main
The pioneering studies of Alan F. Williams and colleagues in the 1980s1 laid the foundations for our current understanding of the roles of the immunoglobulin superfamily (IgSF) molecules as recognition molecules in the immune system. The immunoglobulin domain is a highly versatile fold that can be used to bind an almost infinite array of molecular structures, as illustrated by antibodies and T-cell receptors. The 'immunoglobulin-type' (I-type) lectins are a discrete subset of the IgSF that exploit the remarkable structural diversity of glycans in their recognition functions2,3. The Siglecs (sialic-acid-binding immunoglobulin-like lectins) are the best characterized I-type lectins2,3,4,5,6,7,8,9. They are type 1 membrane proteins displaying an amino-terminal V-set immunoglobulin domain that binds sialic acid and variable numbers (16 in the case of sialoadhesin) of C2-set immunoglobulin domains. They are categorized into two subsets on the basis of their sequence similarity and evolutionary conservation. Sialoadhesin (also known as Siglec-1 and CD169), CD22 (also known as Siglec-2), myelin-associated glycoprotein (MAG; also known as Siglec-4) and the recently discovered Siglec-15 (T. Angata, personal communication) are quite distantly related (∼25–30% sequence identity) and have clear orthologues in all mammalian species examined. In comparison, the CD33-related Siglecs share ∼50–99% identity but seem to be evolving rapidly by multiple processes, including gene duplication, exon shuffling, exon loss and gene conversion10 (Box 1). This has resulted in important differences in the repertoires of CD33-related Siglecs among mammalian species. Initial analyses of the genomes of fish, amphibians and birds indicate that, whereas typical CD33-related Siglecs are absent, a clear orthologue of MAG is present in all three taxa11. In humans, there are nine CD33-related Siglecs and one Siglec-like protein, whereas in mice there are five CD33-related Siglecs. So, it is difficult to assign orthologues, which has required the use of different numbering systems for the human and mouse CD33-related Siglecs (Fig. 1).
Sialic-acid-binding immunoglobulin-like lectins (Siglecs) are type 1 membrane proteins containing an amino-terminal V-set immunoglobulin domain that mediates sialic-acid recognition and varying numbers of C2-set immunoglobulin domains. Siglecs can be divided into two groups based on sequence similarity and evolutionary conservation. The CD33-related Siglecs differ in composition between species, share high sequence similarity in their extracellular regions and frequently contain conserved tyrosine–based signalling motifs in their intracellular domains. By contrast, there are orthologues, in all mammals examined, of sialoadhesin, CD22, myelin-associated glycoprotein (MAG) and Siglec-15 and they exhibit lower sequence similarity. Siglec-14 and Siglec-H have been shown to associate with DAP12 through a charged residue in their transmembrane domains. Electron microscopy has shown that purified sialoadhesin is a tadpole-shaped molecule of ∼40 nm in which the carboxy-terminal immunoglobulin-like domains are arranged into a globular shape128. The cell-expression patterns are indicated on the basis of antibody labelling, except for Siglec-14 and Siglec-15, which have not yet been determined. The brackets indicate low levels of expression. Siglec-13 is present in baboons and chimpanzees and is specifically deleted in humans. Siglec-12 in humans has lost the ability to bind sialic acids and is, hence, designated as Siglec-XII (not shown). B, B cells; Ba, basophils; cDCs, conventional dendritic cells; Eo, eosinophils; GRB2, growth-factor-receptor-bound protein 2; ITIM, immunoreceptor tyrosine-based inhibitory motif; Mac, macrophages; Mo, monocytes; MyP, myeloid progenitors; N, neutrophils; ND, not determined; NK, natural killer cells; OligoD, oligodendrocytes; pDCs, plasmacytoid dendritic cells; Schw, Schwann cells; Troph, trophoblasts.
With the exception of MAG and Siglec-6, Siglec expression has been found mainly in the haematopoietic and immune systems (Fig. 1). Some are expressed in a highly cell-type-restricted pattern. For example, sialoadhesin is a macrophage-specific adhesion molecule and CD22 is a well-characterized B-cell inhibitory receptor that regulates multiple B-cell functions, including cellular activation thresholds and survival. In general, the CD33-related Siglecs show more complex expression patterns in the innate immune system in both humans and mice (Fig. 1). With the exception of resting T cells, most cell types in the human and mouse immune systems express at least one Siglec and others express several. Each Siglec has a unique specificity for sialylated ligands, making it more probable that each protein mediates a distinct, partially overlapping function. CD22 and most CD33-related Siglecs have one or more cytosolic immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Classically, receptors with ITIMs function as inhibitory receptors and suppress activation signals that emanate from receptors associated with immunoreceptor tyrosine-based activation motifs (ITAMs) through the recruitment of tyrosine and inositol phosphatases12. By contrast, mouse CD33 and Siglec-H, and human Siglec-14 and Siglec-15 lack ITIMs. Two of these non-ITIM-containing Siglecs (Siglec-H and Siglec-14) have been shown to interact with DAP12 (also known as KARAP)13,14, an ITAM-containing adaptor that triggers both activating and inhibitory signalling15. Siglec-13, which is present in other primates but is specifically deleted in humans9, also seems to recruit DAP12 (A.V., unpublished observations).
Taken together, it is clear that Siglecs in the immune system have the potential to mediate both cell–cell interactions and signalling functions. However, defining their precise functions and determining which ligands are biologically relevant pose an important challenge. This is beginning to be tackled using a combination of experimental approaches, including the production of genetically manipulated mice, biochemical analyses of ligand recognition and dissection of signalling pathways. In particular, several recent studies using mice that lack CD22, CD22 ligands or both, as well as mice expressing mutant forms of CD22 that cannot bind sialylated glycans, have begun to shed light on the complex factors involved. These have also provided a conceptual framework for understanding how the less well-characterized CD33-related Siglecs may contribute to regulation of leukocyte functions, as revealed in a recent study of Siglec-F-deficient mice107. Sialoadhesin (recognized by the antibody MOMA-1) is well known as a macrophage-specific marker and adhesion molecule but its biological functions have remained enigmatic. However, several recent studies of sialoadhesin-deficient mice have shown an unexpected role of this receptor in modulating immune and inflammatory responses. New data are available on the endocytic functions of Siglecs and their interactions with various sialylated pathogens that could be important in both host defence and pathogenicity. Finally, there is emerging evidence that CD33-related Siglecs have undergone significant changes during human evolution. In this Review, we discuss how these recent advances have significantly furthered our understanding of the roles of Siglecs in the immune system and wherever possible we attempt to relate these functions to glycan recognition and physiology.
Sialic-acid recognition by Siglecs
The mammalian glycome contains numerous sialylated glycans that can be potentially recognized as ligands by Siglecs (Box 2). It is assumed that this recognition is important for modulating the functions of Siglecs as regulators of adhesion, cell signalling and endocytosis5,6,7,8,9. In general, Siglecs show low affinity (a Kd of 0.1–3 mM) for the sialic acid N-acetylneuraminic acid (Neu5Ac) α2–3 and α2–6 linkages to galactose (Neu5Acα2–3Gal and Neu5Acα2–6Gal) (Fig. 2) that are commonly found as terminal sequences on glycans of glycoproteins and glycolipids of most mammalian cells16,17, and Siglecs have an overlapping specificity for such sialosides (sialic-acid-containing glycans). However, when examined for their ability to recognize a diverse set of natural sialoside structures found in mammalian species, each Siglec shows a characteristic specificity profile (Fig. 3). CD22 is unique in having a strong preference for Neu5Acα2–6Gal and Neu5Gcα2–6Gal structures18,19. Siglec-7 and Siglec-11 prefer sialosides with the Neu5Ac(α2–8)Neu5Ac structure20,21.
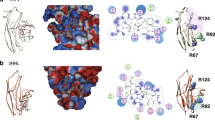
a | Sialic acid in mammals is commonly modified at the R and R′ positions with the substituents indicated. There are many other kinds of sialic acids in vertebrates, but apart from the commonly found forms shown here, Siglec (sialic-acid-binding immunoglobulin-like lectin) interactions with these have not been studied systematically. b | Two common sialoside sequences recognized as low-affinity ligands by many Siglecs are shown. c | Structure of the V-set domain of sialoadhesin (gold and grey) complexed with Neu5Acα2–3Gal (shown with turquoise carbons). Side chains of amino acids that make direct contact with the bound ligand are shown with purple carbons. The C–C´ loop that is implicated in fine specificity of Siglec binding is shown in orange. d | Structure of the V-set domain of Siglec-7 (gold and grey) complexed with Neu5Ac (shown with turquoise carbons). Side chains of amino acids that make direct contact with the bound ligand are shown with purple carbons. The C–C´ loop that is implicated in fine specificity of Siglec binding is shown in orange. Gal, galactose; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid. Images in parts c and d are reproduced with permission from Ref. 28 © (2003) American Society for Biochemistry and Molecular Biology.
A comparison of the sialoside specificities, obtained from glycan array analysis and competitive binding experiments, for several sialic-acid-binding immunoglobulin-like lectins (Siglecs) against selected sialoside sequences found in mammalian glycoproteins and glycolipids17,22,23,24 (see also the Functional Glycomics web site). Despite the general low affinity of Siglecs towards the common mammalian sialoside structures containing the N-acetylneuraminic acid (Neu5Ac) α2–6 and α2–3 linkages, examination of the binding of each individual Siglec to a range of common sialoside structures shows that there is a unique sialoside specificity profile that is characteristic to each Siglec. +++, strong binding; ++, moderate binding; +, low binding; +/−, detectable binding; 0, no detectable binding; GlcNAc, N-acetylglucosamine; ND, not determined; Neu5Gc, N-glycolylneuraminic acid.
Several Siglecs (such as Siglec-8 and Siglec-9) have differential specificity for sialosides that contain both sialic acid and sulphate, with the position of the sulphate being an important determinant of specificity22,23,24,25. In addition, Siglecs have different specificities for the many sialic acid species found in nature (Fig. 2). Of particular interest is the evolutionary loss of N-glycolylneuraminic acid (Neu5Gc) in humans, as Neu5Gc is the preferred ligand for at least some Siglecs in the closely related great apes26.
The ligand-bound crystal structures of sialoadhesin and Siglec-7 offer insights into the structural basis for Siglec specificity27,28,29,30,31. In both cases, sialic acid is bound to a shallow pocket in the N-terminal immunoglobulin domain of the Siglec, with the negatively charged carboxyl group of sialic acid forming an important salt bridge with a conserved essential arginine (Fig. 2). As shown in domain-swapping experiments20, the highly variable C–C´ loop (residues 70–75 in Siglec-7) of the V-set domain is a key determinant of Siglec specificity. In the crystal structure of Siglec-7 complexed with the Neu5Acα2–8Neu5Ac-containing ganglioside known as GT1b, the C–C´ loop undergoes a marked conformational change30. Although binding between natural sialosides and Siglecs is relatively weak, increased binding affinity has been seen for sialoadhesin, MAG and CD22 using sialic acid analogues that have aromatic substituents at the C-9 position of the monosaccharide29. These have been exploited for the creation of high-affinity ligand-based probes of Siglec function29,31,32,33,34,35.
Cis and trans ligands. The local concentration of sialic acids on surfaces of immune cells is very high; for example, on B cells it has been estimated to exceed 100 mM36. As a consequence of this, and the fact that Siglecs usually recognize sialoside sequences that are commonly found on mammalian cells, Siglec binding sites are typically 'masked' by cis interactions with other glycan ligands expressed on the same cell37,38,39,40. This indicates that interactions with cis ligands may dominate over interactions with trans ligands in modulating the biological activities of Siglecs7,9 (Fig. 4). One exception to this rule is sialoadhesin, which owing to its extended structure is thought to project its sialic-acid-binding site away from the plasma membrane, which reduces its cis interactions41. Despite the likely importance of cis-ligand interactions in Siglec function, they do not necessarily prevent the binding of ligands in trans. CD22 on B cells can redistribute to the site of contact with another cell expressing CD22 ligands36. Moreover, high-affinity synthetic sialoside probes can out-compete cis ligands for binding to CD22 on native B cells34. These results show that cis ligands downregulate, but do not preclude, binding of ligands in trans, and that equilibrium-based binding of Siglecs to trans ligands can occur dynamically in the presence of cis ligands. The biological significance of CD22 trans-interactions was explored by Lanoue et al. who showed that B-cell activation in response to antigen-presenting cells is suppressed if antigen and the CD22 ligand are expressed on the same cell42. This could be important for preventing autoantibody production by self-reactive B cells.
a | Most sialic-acid-binding immunoglobulin-like lectins (Siglecs) are masked at the cell surface owing to cis interactions with abundantly expressed sialic acids. Following exposure of cells to sialidase, which cleaves the cis-interacting Siglec ligands, or in some cases following cellular activation, Siglecs become unmasked, which allows them to make interactions with ligands in trans. b | Even when Siglecs are masked by cis interactions, trans interactions might occur during an encounter with another cell or a pathogen expressing higher affinity ligands that can out-compete the cis interactions.
Siglec-7 is a natural killer (NK)-cell inhibitory receptor43,44, which is masked by cis ligands that may include the preferred Neu5Acα2–8Neu5Ac-containing glycan45. In NK-cell cytotoxicity experiments with target cells engineered to overexpress this glycan structure, a small Siglec-7-dependent reduction in NK-cell cytotoxicity was observed and this was increased when NK cells were pre-treated with sialidase, which cleaves the cis-interacting ligands from the cell surface46. Such trans-interactions could contribute to the suppression of NK-cell cytotoxicity in tissues, such as the nervous system, in which the Neu5Acα2–8Neu5Ac-containing glycans are abundantly expressed.
In common with many glycan-binding proteins, high-avidity interactions of Siglecs require clustering of both receptors and ligands. For membrane receptors, clustering can be achieved by lateral diffusion in the plasma membrane or by the formation of multimeric complexes. For the glycan ligands, clustering can occur by presentation on suitable carriers, either protein or lipid, and/or by clustering on another cell surface. The resulting 'counter-receptors' have the potential to be recognized by glycan-binding proteins with high avidity and selectivity47. Although information on the nature of physiologically important ligands and counter-receptors of the Siglec family remains fragmentary, some insights have been obtained for CD22. B cells from mice deficient in the sialyltransferase ST6GAL1, which transfers sialic acids to galactose in α2–6 linkages to produce the preferred ligands for CD22, were devoid of cis ligands, which indicates that this sequence is required for binding by CD22 (Ref. 48). A CD22–immunoglobulin Fc fusion protein has been shown to bind to CD45, serum IgM and many other B-cell (and T-cell) glycoproteins in vitro, and this implicates CD45 and IgM as candidate cis glycoprotein counter-receptors for CD22 on B cells49,50,51. However, using B cells that are metabolically labelled with a sialic-acid analogue containing a photo-activatable crosslinker, Han et al. found that CD45 and IgM are not preferentially recognized as cis ligands on the surface of the intact cell33. Instead, CD22 interacted preferentially in cis with glycans of neighbouring CD22 molecules. This is consistent with earlier crosslinking experiments52,53, which also showed the formation of homomultimeric CD22 complexes involving protein–protein interactions. The basis for this selectivity may stem from the preferential concentration of CD22 in clathrin domains35,54,55. These studies underscore the potential difficulty in establishing the in situ ligands of Siglecs in their native context.
Pathogen ligands and innate immunity. Over 20 pathogenic microorganisms are known to have evolved the capacity to synthesize or capture sialic acids from their hosts and incorporate these into their own glycoconjugates. Examples include well-known bacterial pathogens, such as Neisseria meningitidis, Haemophilus influenzae, group B Streptococcus, Campylobacter jejuni, and several strains of Escherichia coli that cause infant meningitis, septicaemia, respiratory infections, diarrhoea and various other conditions56. Sialylation of glycoconjugates in these pathogens seems to be crucial for their survival in the mammalian host, possibly serving as molecular mimics of host cell surfaces to avoid immune attack57. It is also widely assumed that negatively charged sialic acids could serve to reduce pathogen interactions with the host by electrostatic repulsion, and/or by inhibiting the alternative pathway of complement activation. So far, comparatively little attention has been paid to the possibility that pathogen sialic acids are important for actually promoting infection or mediating an innate immune response by attachment to Siglecs. One clear example of this is porcine reproductive and respiratory syndrome virus (PRRSV), which is a sialylated enveloped virus that infects pig alveolar macrophages in a sialic-acid and sialoadhesin-dependent manner58,59. Sialoadhesin and several CD33-related Siglecs can interact with sialic acids on N. meningitidis, C. jejuni, group B Streptococcus and Trypanosoma cruzi60,61,62,63 (Table 1). Siglec-dependent uptake of these pathogens could potentially benefit the host by promoting pathogen destruction and antigen presentation. It has also been suggested, but not proved, that sialylated pathogens modulate leukocyte activation through ITIM-mediated signalling of CD33-related Siglecs, thereby benefiting the pathogen by dampening inflammatory and immune responses8,60,62,63.
Sialoadhesin as a modulator of immune responses
Sialoadhesin was discovered as a sialic-acid-dependent macrophage adhesion molecule64. It is one of the largest members of the IgSF with an extracellular region made up of 17 immunoglobulin domains65, a feature that is well conserved in mammals. Unlike most Siglecs, sialoadhesin lacks tyrosine-based signalling motifs and its cytoplasmic tail is poorly conserved, which suggests a primary role as a binding partner in cell–cell interactions, rather than in cell signalling.
There is increasing evidence to support a contribution for sialoadhesin in the pro-inflammatory functions of macrophages. Sialoadhesin is constitutively expressed on subpopulations of tissue-resident macrophages66,67, and is rapidly upregulated by inflammatory macrophages. In proliferative glomerulonephritis, the expression of sialoadhesin correlates with proteinuria68,69, and in individuals infected with HIV and that carry high viral loads, the sialoadhesin gene is induced up to tenfold in circulating monocytes70. It was recently shown that under normal conditions, sialoadhesin-deficient mice show only subtle alterations in the haematopoietic and immune systems, but in a model of peptide-induced experimental autoimmune uveoretinitis, the deficient mice showed reduced retinal inflammation, and T cells isolated from draining lymph nodes showed lowered proliferative responses in vitro71. Furthermore, in two genetically determined models of peripheral and central nervous system demyelination, disease in sialoadhesin-deficient mice was ameliorated and numbers of infiltrating CD8+ T cells and macrophages at the sites of inflammation were reduced72,73. These new findings are consistent with a potentially important role of sialoadhesin in modulating T-cell function and activation during immune responses. An additional possibility is that sialoadhesin functions as a phagocytic receptor to clear sialylated pathogens.
Although the cellular and molecular bases for these effects require further investigation, sialoadhesin can mediate both sialic-acid-dependent and sialic-acid-independent interactions with cells of the immune system. Sialic-acid-dependent sialoadhesin interactions might be mediated by mucin-like molecules presenting high densities of sialylated O-linked glycans. For example, the sialomucins CD43 and mucin-1 were previously identified as putative T-cell and breast-cancer cell counter-receptors for sialoadhesin, respectively74,75. Sialic-acid-independent sialoadhesin interactions could involve the mannose receptor and macrophage galactose-type N-acetylgalactosamine-specific lectin 1 (MGL1). Both of these membrane lectins are expressed on dendritic cells (DCs) and have been shown to bind sialoadhesin extracted from lymphoid tissues76,77. Although DCs themselves do not normally express sialoadhesin, it can be induced on human monocyte-derived DCs following exposure to rhinoviruses in vitro78. Interestingly, these DCs were poor stimulators of T cells in mixed lymphocyte reactions, a feature that was partly attributed to the expression of sialoadhesin78. It is possible that when sialoadhesin is expressed by macrophages it is immunostimulatory, whereas on DCs it is immunosuppressive.
Role of CD22 and its ligands
CD22 is a well-documented regulator of B-cell signalling, homeostasis and survival7,8,9,79. This Siglec is best known for helping to set a threshold for antigen-induced activation of B cells80, an activity that involves as many as six tyrosine-based motifs in the cytoplasmic domain of CD22, including three ITIMs. B-cell receptor (BCR) ligation leads to increased phosphorylation of the ITIMs of CD22 by the SRC-family kinase LYN, which results in the recruitment of SHP1 (SRC homology 2 (SH2)-domain-containing protein tyrosine phosphatase 1) and the downregulation of BCR signalling8,79. However, this oversimplifies the complexity of CD22 signalling, as it can also recruit positive effectors of cell activation, including GRB2 (growth-factor-receptor-bound protein 2), SHC (SH2-domain-containing transforming protein C), PI3K (phosphoinositide 3-kinase) and PLCγ2 (phospholipase Cγ2)79. This results in activation of alternative signalling pathways that contribute to the regulation of B-cell activation. The impact of CD22 on these pathways probably depends on the manner of B-cell activation. Ligation of the BCR with either antigen or IgM-specific antibody, or simultaneous ligation of the BCR and CD40 (with IgM-specific and CD40-specific antibodies) result in the differential phosphorylation of CD22 tyrosine-based motifs both quantitatively and qualitatively81,82. Furthermore, CD22 does not seem to affect B-cell signalling when activated by ligation of cell-surface IgG83. A detailed molecular understanding of the role of CD22 will continue to evolve as B-cell signalling pathways become better defined8,79.
Recent work on CD22 has provided important insights into how sialic-acid recognition can modulate its signalling functions. B cells of CD22-deficient mice exhibit hyperimmune responses in vitro and in vivo8,79, consistent with the loss of negative regulation by ITIMs of CD22. Several CD22 functions, including BCR-dependent proliferation and B-cell turnover rates, depend on the ligand-binding function of CD22, as shown using mice that carry knock-in mutations of CD22 that ablate its ability to bind sialic acid84. In contrast to CD22-deficient mice, ST6GAL1-deficient mice (which lack CD22 ligands)35,55,85 exhibit hypo-immune responses. B cells from mice that are deficient in both CD22 and ST6GAL1 behave similarly to those from CD22-deficient mice, which indicates that the immunodeficiency of ST6GAL1-deficient mice depends on the presence of CD22 (Refs 35, 55, 85). Following BCR ligation in vitro, the immunodeficiency caused by the absence of cis ligands in ST6GAL1-deficient mice is manifest by reduced B-cell proliferation and calcium flux, and increased CD22 phosphorylation and recruitment of SHP1 (Refs 35, 55). Grewal et al. recently showed that ablating St6gal1 in the LYN-deficient mouse model of lupus abrogated autoimmune responses55, which indicates the therapeutic implications of inducing a CD22-ligand deficiency.
Insights into how the absence of CD22 cis ligands reduces B-cell signalling have been obtained by assessing the microdomain localization of CD22 in the membrane, relative to that of the BCR35,55 (Fig. 5). In resting B cells, there is minimal colocalization of CD22 with the BCR. Most of the CD22 molecules (∼80%) are associated with clathrin domains, which is consistent with the presence of cytoplasmic tyrosine-based motifs for the clathrin adaptor protein AP50 (Ref. 54). By contrast, the BCR is minimally associated with clathrin domains. Following B-cell activation, the BCR moves into activation rafts, which subsequently fuse with clathrin domains before they are endocytosed86,87. Because CD22 is excluded from activation rafts, it is probable that CD22 exerts its regulation of BCR signalling in the fused raft/clathrin domains34,55,86,87. In ligand-deficient mice, there is no change in the localization of CD22. However, the amount of cell-surface IgM that is colocalized with CD22 increases twofold (from 20–25% to 40–50%), which is associated with a dramatic shift in the distribution of IgM to the raft/clathrin domains. This shift in BCR localization also results in increased endocytosis and reduced half-life of IgM35,55. The redistribution and reduced half-life of IgM do not occur in mice deficient in both CD22 and ST6GAL1, indicating they are mediated by CD22, and are not due to another effect of the ST6GAL1 deficiency. So, for ligand-deficient mice, the increased colocalization of the BCR and CD22 in fused raft/clathrin domains of resting B cells probably accounts for the reduced BCR signalling mediated by CD22 (Fig. 5). These results also indicate that cis ligands of CD22 reduce BCR localization in raft/clathrin domains of resting B cells of wild-type mice by an as-yet-undefined mechanism.
In resting B cells, CD22 is mainly localized in clathrin-coated pits, whereas the B-cell receptor (BCR) shows minimal association with these microdomains. In the absence of CD22-ligand binding (such as in ST6GAL1-deficient mice), the BCR shows increased colocalization with CD22, which is coincident with the reduction of BCR signalling. This mainly occurs in fused raft/clathrin domains that typically form following BCR ligation before endocytosis of the BCR.
In addition to the effect on BCR signalling, deficiency in CD22-ligand binding leads to reduced levels of cell-surface CD22 and IgM, increased apoptosis and B-cell turnover, and a reduction in the number of marginal-zone B cells84. These properties are also observed in CD22-deficient mice, which suggests that they are regulated by CD22–ligand interactions35,55,84,85,88. So, it is probable that other important roles of both cis and trans ligands in CD22 function are likely to be revealed using appropriate mouse models.
Functions mediated by CD33-related Siglecs
The CD33-related Siglecs are mainly expressed by mature cells of the innate immune system, such as neutrophils, eosinophils, monocytes, macrophages, NK cells, DCs and mast cells (Fig. 1). CD33 itself is well known as a marker of myeloid progenitor cells, indicating a potential role for CD33 in the regulation of cellular proliferation and/or differentiation. Other CD33-related Siglecs seem to be expressed at later stages of haematopoiesis89,90,91. Numerous studies point to important roles of CD33-related Siglecs in modulating leukocyte behaviour, including inhibition of cellular proliferation92,93, induction of apoptosis94,95, inhibition of cellular activation96,97,98,99,100, induction of pro-inflammatory cytokine secretion101 and, in the case of Siglec-H on plasmacytoid DCs (pDCs), suppression of interferon-α (IFNα) production13. In general, these functions have been defined using selected Siglecs (Fig. 6) and the extent to which they can be extrapolated to the other CD33-related Siglecs is unknown. The signalling pathways are poorly understood but in most cases are assumed to involve the ITIM and ITIM-like motifs and recruitment of tyrosine phosphatases (Fig. 6). It should be emphasized that many of these studies have been carried out using Siglec-transfected cells and/or antibody crosslinking of cell-surface proteins, and the activities need to be confirmed in more physiological systems.
On phosphorylation of cytoplasmic tyrosine–based signalling motifs by SRC-family tyrosine kinases, sialic-acid-binding immunoglobulin-like lectins (Siglecs) recruit and activate SRC homology 2 (SH2)-domain-containing proteins, notably the tyrosine phosphatases SHP1 (SH2-domain–containing protein tyrosine phosphatase 1) and SHP2 or the SOCS3 (suppressor of cytokine signalling 3) protein. This initiates a range of functional activities that are indicated for the Siglecs listed. In the case of Siglecs without cytosolic signalling motifs, charge-dependent transmembrane region interactions with DAP12 can provide ITAM (immunoreceptor tyrosine-based activation motif)-based signalling functions that are typically initiated by the recruitment and activation of spleen tyrosine kinase (SYK). ↑, increased; ↓, decreased; IFNα, interferon-α.
An important function of CD33-related Siglecs is their ability to regulate cell growth and survival, either by inhibition of proliferation or induction of apoptosis. Inhibition of proliferation was first shown for CD33 and Siglec-7 using both normal haematopoietic cells and myeloid leukaemic cells92,93 and it was recently confirmed using transfected Ba/F3 cells102,103. With the Ba/F3 cells, inhibition of cytokine-dependent proliferation depended on crosslinking Siglecs with primary and secondary antibodies and required an intact ITIM102,103. Induction of apoptotic and non-apoptotic cell death has been shown for human eosinophils and neutrophils through antibody-induced ligation of Siglec-8 and Siglec-9, respectively94,95. Interestingly, cell-death induction by these Siglecs is enhanced in the presence of cytokines that normally promote survival, which suggests a complex interplay between cytokine receptor and Siglec signalling pathways104. Regulation of ITAM-dependent cellular activation by CD33-related Siglecs has been shown in various cell types, including transfected T cells, mast cell lines and myeloid cell lines. Interestingly, both primary human T cells and Jurkat cells, which normally lack significant levels of CD33-related Siglecs, showed inhibition of T-cell-receptor-dependent activation following overexpression of Siglec-5, Siglec-7 or Siglec-9 (Refs 99, 105). In comparison, chimpanzee T cells express several CD33-related Siglecs and this may account for their lower proliferative responses compared with human T cells105.
The most direct evidence for a role of CD33-related Siglecs in the modulation of leukocyte functions has been provided by studies of mice lacking Siglec-F107, which is a functionally convergent paralogue of human Siglec-8 and is expressed by eosinophils23,106 and by activated T cells. In a model of induced lung allergy, these mice show increased bone-marrow, blood and tissue eosinophilia, which is consistent with an inhibitory role of CD33-related Siglecs in controlling leukocyte expansion during inflammatory responses107. Taken together, these data indicate the possible existence of a negative-feedback loop that controls allergic responses of eosinophils and helper T cells, through Siglec-F and upregulated expression of Siglec-F ligands in the inflamed tissue.
CD33-related Siglecs can also function as endocytic receptors that could be important in the clearance of sialylated antigens and/or in promoting or inhibiting antigen presentation90,91,108,109,110. Their endocytic capacity can also be exploited for therapy. For example, gemtuzumab ozogamicin (Mylotarg; UCB and Wyeth-Ayerst Laboratories (Wyeth)) is a humanized CD33-specific monoclonal antibody coupled to the potent antibiotic calicheamicin-γ1, which is currently being used for the treatment of relapsed acute myeloid leukaemia (AML) following chemotherapy. Recent screens of CD33-related Siglec expression by AML cells have shown that several are expressed at variable levels and may therefore provide additional targets for the treatment of haematological diseases90,91.
Signalling by CD33-related Siglecs. Most CD33-related Siglecs have two conserved cytoplasmic tyrosine-based motifs, comprising a membrane-proximal ITIM and a membrane-distal ITIM-like motif (consensus sequence (Glu/Asp)-Tyr-X-Glu-(Val/Ile)-(Arg/Lys); where X denotes any amino acid) (Fig. 1). The distal motif was originally described111 as being similar to a signalling motif in the SLAM (signalling lymphocytic activation molecule) family112 of receptors that recruit SAP (SLAM-associated protein) and/or its homologue EAT2 (Ewing's sarcoma-associated transcript 2). However, more recent studies on the consensus sequence for SAP and EAT2 binding (Thr-Ile-Tyr-X-X-(Val/Ile))112 make it seem unlikely that CD33-related Siglecs interact with these adaptors. Mutagenesis experiments with CD33-related Siglecs have shown that the ITIM dominates over the ITIM-like motif, both for the recruitment of SHP1 and SHP2 and for inhibitory signalling functions96,97,98,100,113,114. However, the ITIM-like motif was required for optimal recruitment of SHP1, but not of SHP2, and could therefore be important in fine-tuning downstream signalling from CD33-related Siglecs.
The ITIMs of CD33-related Siglecs are important for other functions, including the suppression of Siglec-dependent adhesion to sialylated ligands and endocytosis91,98,100,110,113. The degree of tyrosine phosphorylation is likely to be crucial in determining which function predominates. Robust binding to SHP1 and SHP2 requires tyrosine phosphorylation of both the ITIM and ITIM-like motif. However, following mutation of both tyrosines to alanine, Siglec-5 was capable of weak SHP1 binding and activation and was still a potent inhibitor of cellular activation100. By contrast, mutation of the ITIM alone reversed the suppression of Siglec-dependent adhesion and this correlated with the loss of SHP2 binding98,100.
Orr et al. have recently shown that suppressor of cytokine signalling 3 (SOCS3) can bind efficiently to the phosphorylated ITIMs of CD33 and Siglec-7 and compete with SHP1 and SHP2 for ITIM-dependent inhibition of cytokine-induced proliferation102,103. Recruited SOCS3 was shown to function as an E3 ligase, leading to proteasome-dependent degradation of both Siglec and SOCS3. As SOCS3 is a key negative regulator of cytokine signalling in myeloid cells115, CD33–SOCS3 interactions could affect both SOCS3 and Siglec-dependent inhibitory functions. Taken together, these findings indicate a finely balanced mechanism of leukocyte activation and growth regulation involving CD33-related Siglecs, SHP1, SHP2 and SOCS3 proteins (Fig. 6).
Siglec-H is coupled to DAP12. Siglec-H is a CD33-related Siglec that is expressed by mouse and rat pDCs13,109,116. This receptor can mediate endocytosis and cross-presentation of antigens109 and can also function as a negative regulator of IFNα production by pDCs13. This observation is surprising because Siglec-H lacks ITIM-like motifs and depends on the presence of the ITAM-containing adaptor DAP12 for cell-surface expression13. However, there is growing evidence that under certain circumstances ITAM-associated receptors can mediate inhibitory signalling through poorly characterized mechanisms15. So far, Siglec-H has not been shown to bind sialic acids109, and the rat orthologue has a mutation in the arginine residue that is required for sialic-acid binding10. Strictly speaking, therefore, Siglec-H may not fulfil the criteria to be defined as a bona fide Siglec117. One interesting possibility is that this pDC-restricted receptor has evolved from sialic-acid binding to function as a pattern-recognition molecule that binds viral or other pathogen ligands and delivers them to endosomal compartments for subsequent triggering of Toll-like receptor (TLR)-dependent cytokine responses and antigen presentation118. So far, there are no reports addressing the possibility that the more conserved C2-set immunoglobulin domains of CD33-related Siglecs might mediate protein–protein interactions that are important in immune functions.
Siglec-5 is paired with Siglec-14, a DAP12-coupled receptor. Several inhibitory receptors of the immune system are paired with activating counterparts that share highly related extracellular domains but differ in the transmembrane and cytoplasmic regions. Paired activating receptors often have a positively charged residue within the transmembrane region that is required for binding to the ITAM-containing adaptors, such as DAP12 and the Fc receptor γ-chain, or to the DAP10 adaptor that contains the sequence Tyr-X-X-Met (where X is any amino acid), which recruits PI3K119. As shown for the NK-cell receptor Ly49H, which binds the MHC-like protein m157 of mouse cytomegalovirus120,121, some paired activating receptors may have evolved as a counter-strategy to bind host-derived pathogen ligands. Although Siglecs were not previously thought to include paired receptors, recent data indicate that the inhibitory receptor Siglec-5 is paired with a putative activating receptor, Siglec-14, and both seem to be expressed by myeloid cells in a coordinated manner14. These proteins share more than 99% sequence identity in their first two immunoglobulin domains and then diverge in the rest of their coding sequences. Siglec-14 has an arginine residue in its transmembrane region that is required for its association with DAP12 (Ref. 14). Siglec-5 and Siglec-14 would therefore be expected to deliver opposing signals through ITIM- and ITAM-dependent pathways, respectively. A different arginine residue (of the first immunoglobulin domain) that is required for sialic-acid recognition by both Siglec-5 and Siglec-14 is present in humans (but is mutated in almost all great-ape alleles), indicating that these two proteins may work in a cooperative manner, balancing activating and inhibitory signalling through sialic-acid recognition14. Indeed, repeated gene-conversion events occurred between the 5′ regions of the Siglec5 and Siglec14 genes in each primate lineage, assuring maintenance of a paired receptor status for sialic-acid binding.
Concluding remarks
Siglecs are emerging as important regulators of the immune system. What links this family of proteins at a molecular level is their ability to bind sialic acids in a range of glycoconjugates, both in cis and in trans. Challenges for the future are to understand in precise terms which ligands and counter-receptors are important for mediating the biological functions of Siglecs, to elucidate the role of sialic-acid recognition in Siglec biology and to dissect the signalling pathways that are triggered. Siglecs are also recognized as endocytic receptors and this function is likely to be regulated by the same tyrosine motifs that regulate signalling. The interplay between the regulation of receptor signalling and endocytosis may prove to be a fruitful area of investigation for the future. A related challenge will be to understand the impact of pathogen sialylation on Siglec-mediated host immune responses, an issue that may give insights into the evolutionary pathways that have led to the diversification of this family. With growing data linking inhibitory receptor polymorphisms and autoimmune disease, studies of Siglec polymorphisms among human populations are clearly warranted. Finally, the development of Siglec-specific agonists and antagonists may provide new approaches to the treatment of certain autoimmune and inflammatory conditions.
References
Williams, A. F. & Barclay, A. N. The immunoglobulin superfamily — domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405 (1988).
Powell, L. D. & Varki, A. I-type lectins. J. Biol. Chem. 270, 14243–14246 (1995).
Angata, T. & Brinkman-Van der Linden, E. I-type lectins. Biochim. Biophys. Acta 1572, 294–316 (2002).
Kelm, S. et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 (1994). This paper established the existence of the Siglec family, initially described as the sialoadhesin family.
Crocker, P. R. & Varki, A. Siglecs, sialic acids and innate immunity. Trends Immunol. 22, 337–342 (2001).
Crocker, P. R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell–cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002).
Crocker, P. R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5, 431–437 (2005).
Nitschke, L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr. Opin. Immunol. 17, 290–297 (2005).
Varki, A. & Angata, T. Siglecs — the major subfamily of I-type lectins. Glycobiology 16, 1R–27R (2006).
Angata, T., Margulies, E. H., Green, E. D. & Varki, A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl Acad. Sci. USA 101, 13251–13256 (2004). This extensive bioinformatics analysis on rodent and primate genome sequences establishes that the CD33-related Siglecs are rapidly evolving and exhibit striking differences in repertoire between mammalian species.
Angata, T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol. Divers. 10, 555–566 (2006).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Blasius, A. L., Cella, M., Maldonado, J., Takai, T. & Colonna, M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 107, 2474–2476 (2006).
Angata, T., Hayakawa, T., Yamanaka, M., Varki, A. & Nakamura, M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 20, 1964–1973 (2006).
Hamerman, J. A. & Lanier, L. L. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006, re1 (2006).
Bakker, T. R., Piperi, C., Davies, E. A. & Merwe, P. A. Comparison of CD22 binding to native CD45 and synthetic oligosaccharide. Eur. J. Immunol. 32, 1924–1932 (2002).
Blixt, O., Collins, B. E., van den Nieuwenhof, I. M., Crocker, P. R. & Paulson, J. C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278, 31007–31019 (2003).
Powell, L. D., Sgroi, D., Sjoberg, E. R., Stamenkovic, I. & Varki, A. Natural ligands of the B cell adhesion molecule CD22β carry N-linked oligosaccharides with α-2,6-linked sialic acids that are required for recognition. J. Biol. Chem. 268, 7019–7027 (1993). This paper shows that CD22, an IgSF member, is a sialic-acid-binding lectin with strict specificity for α2–6-sialylated glycans.
Kelm, S., Schauer, R., Manuguerra, J. C., Gross, H. J. & Crocker, P. R. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconj. J. 11, 576–585 (1994).
Yamaji, T., Teranishi, T., Alphey, M. S., Crocker, P. R. & Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to α2,8-disialyl and branched α2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 277, 6324–6332 (2002).
Hayakawa, T. et al. A human-specific gene in microglia. Science 309, 1693 (2005).
Bochner, B. S. et al. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 280, 4307–4312 (2005).
Tateno, H., Crocker, P. R. & Paulson, J. C. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 15, 1125–1135 (2005).
Campanero-Rhodes, M. A. et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem. Biophys. Res. Commun. 344, 1141–1146 (2006).
Rapoport, E. M., Pazynina, G. V., Sablina, M. A., Crocker, P. R. & Bovin, N. V. Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 71, 496–504 (2006).
Sonnenburg, J. L., Altheide, T. K. & Varki, A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology 14, 339–346 (2004).
May, A. P., Robinson, R. C., Vinson, M., Crocker, P. R. & Jones, E. Y. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3' sialyllactose at 1.85 Å resolution. Mol. Cell 1, 719–728 (1998). In this paper, the first reported structure of a Siglec complexed with 3' sialyllactose ligand reveals how the immunoglobulin domain can adapt to sialic-acid binding.
Alphey, M. S., Attrill, H., Crocker, P. R. & van Aalten, D. M. High resolution crystal structures of Siglec-7. Insights into ligand specificity in the Siglec family. J. Biol. Chem. 278, 3372–3377 (2003).
Zaccai, N. R. et al. Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure 11, 557–567 (2003).
Attrill, H. et al. Siglec-7 undergoes a major conformational change when complexed with the α2,8-disialylganglioside GT1b. J. Biol. Chem. 281, 32774–32783 (2006).
Attrill, H. et al. The structure of siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design. Biochem. J. 397, 271–278 (2006).
Kelm, S., Gerlach, J., Brossmer, R., Danzer, C. P. & Nitschke, L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 195, 1207–1213 (2002).
Han, S., Collins, B. E., Bengtson, P. & Paulson, J. C. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nature Chem. Biol. 1, 93–97 (2005).
Collins, B. E. et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J. Immunol. 177, 2994–3003 (2006).
Collins, B. E., Smith, B. A., Bengtson, P. & Paulson, J. C. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nature Immunol. 7, 199–206 (2006). This paper, together with references 33, 55 and 85, examines the interplay between CD22, its sialylated ligands and the BCR in B-cell signalling.
Collins, B. E. et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl Acad. Sci. USA 101, 6104–6109 (2004).
Freeman, S. D., Kelm, S., Barber, E. K. & Crocker, P. R. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 85, 2005–2012 (1995). This is the first paper to show that CD33 can function as a sialic-acid binding lectin. Together with reference 38, this paper establishes that Siglecs can be masked when expressed at the cell surface and that unmasking can occur following sialidase-treatment of Siglec-expressing cells.
Hanasaki, K., Varki, A. & Powell, L. D. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by α2–6-sialylation of cellular glycoproteins. J. Biol. Chem. 270, 7533–7542 (1995).
Razi, N. & Varki, A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl Acad. Sci. USA 95, 7469–7474 (1998).
Razi, N. & Varki, A. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9, 1225–1234 (1999).
Munday, J., Floyd, H. & Crocker, P. R. Sialic acid binding receptors (siglecs) expressed by macrophages. J. Leukoc. Biol. 66, 705–711 (1999).
Lanoue, A., Batista, F. D., Stewart, M. & Neuberger, M. S. Interaction of CD22 with α2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur. J. Immunol. 32, 348–355 (2002).
Falco, M. et al. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 190, 793–802 (1999).
Nicoll, G. et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274, 34089–34095 (1999).
Avril, T., North, S. J., Haslam, S. M., Willison, H. J. & Crocker, P. R. Probing the cis interactions of the inhibitory receptor Siglec-7 with α2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of α2,8-sialyltransferase gene expression. J. Leukoc. Biol. 80, 787–796 (2006).
Nicoll, G. et al. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and-independent mechanisms. Eur. J. Immunol. 33, 1642–1648 (2003).
Crocker, P. R. & Feizi, T. Carbohydrate recognition systems: functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 6, 679–691 (1996).
Collins, B. E. et al. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology 12, 563–571 (2002).
Stamenkovic, I., Sgroi, D., Aruffo, A., Sy, M. S. & Anderson, T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2–6 sialyltransferase, CD75, on B cells. Cell 66, 1133–1144 (1991).
Hanasaki, K., Powell, L. D. & Varki, A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J. Biol. Chem. 270, 7543–7550 (1995).
Law, C. L., Aruffo, A., Chandran, K. A., Doty, R. T. & Clark, E. A. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J. Immunol. 155, 3368–3376 (1995).
Zhang, M. & Varki, A. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology 14, 939–949 (2004).
Powell, L. D., Jain, R. K., Matta, K. L., Sabesan, S. & Varki, A. Characterization of sialyloligosaccharide binding by recombinant soluble and native cell-associated CD22. Evidence for a minimal structural recognition motif and the potential importance of multisite binding. J. Biol. Chem. 270, 7523–7532 (1995).
John, B. et al. The B cell coreceptor CD22 associates with AP50, a clathrin-coated pit adapter protein, via tyrosine-dependent interaction. J. Immunol. 170, 3534–3543 (2003).
Grewal, P. K. et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol. Cell. Biol. 26, 4970–4981 (2006).
Vimr, E. & Lichtensteiger, C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10, 254–257 (2002).
Vimr, E. R., Kalivoda, K. A., Deszo, E. L. & Steenbergen, S. M. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68, 132–153 (2004).
Vanderheijden, N. et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 77, 8207–8215 (2003). This paper makes the surprising discovery that the sialylated porcine arterivirus depends on sialoadhesin for infection of macrophages.
Delputte, P. L. & Nauwynck, H. J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 78, 8094–8101 (2004).
Jones, C., Virji, M. & Crocker, P. R. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49, 1213–1225 (2003).
Monteiro, V. G. et al. Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitol. Res. 97, 380–385 (2005).
Avril, T., Wagner, E. R., Willison, H. J. & Crocker, P. R. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 74, 4133–4141 (2006).
Carlin, A. F., Lewis, A. L., Varki, A. & Nizet, V. Group B Streptococcal sialic acids interact with Siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 189, 1231–1237 (2007).
Crocker, P. R. & Gordon, S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 164, 1862–1875 (1986).
Crocker, P. R. et al. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 13, 4490–4503 (1994). This paper, together with reference 18, establishes that members of the IgSF can mediate sialic-acid-dependent adhesion.
Crocker, P. R. & Gordon, S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 169, 1333–1346 (1989).
Hartnell, A. et al. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97, 288–296 (2001).
Ikezumi, Y. et al. The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol. Dial. Transplant. 20, 2704–2713 (2005).
Ikezumi, Y. et al. Histological differences in newly onset IgA nephropathy between children and adults. Nephrol. Dial. Transplant. 20. 2704–2713 (2006).
Pulliam, L., Sun, B. & Rempel, H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 157, 93–98 (2004).
Jiang, H. R. et al. Sialoadhesin promotes the inflammatory response in experimental autoimmune uveoretinitis. J. Immunol. 177, 2258–2264 (2006).
Kobsar, I. et al. Attenuated demyelination in the absence of the macrophage-restricted adhesion molecule sialoadhesin (Siglec-1) in mice heterozygously deficient in P0. Mol. Cell. Neurosci. 31, 685–691 (2006).
Ip, C. W., Kroner, A., Crocker, P. R., Nave, K. A. & Martini, R. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol. Dis. 25, 105–111 (2007).
Nath, D. et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 98, 213–219 (1999).
van den Berg, T. K. et al. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1). J. Immunol. 166, 3637–3640 (2001).
Martinez-Pomares, L. et al. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J. Biol. Chem. 274, 35211–35218 (1999).
Kumamoto, Y. et al. Identification of sialoadhesin as a dominant lymph node counter-receptor for mouse macrophage galactose-type C-type lectin 1. J. Biol. Chem. 279, 49274–49280 (2004).
Kirchberger, S. et al. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J. Immunol. 175, 1145–1152 (2005).
Tedder, T. F., Poe, J. C. & Haas, K. M. CD22: A multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv. Immunol. 88, 1–50 (2005).
Doody, G. M. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995). This is one of the first papers to clearly demonstrate that CD22 functions as an inhibitory receptor of B cells via interactions with SHP1. It also shows that antibody-induced sequestration of CD22 away from the BCR lowers the threshold for B-cell activation by 100-fold.
Fujimoto, M. et al. B cell antigen receptor and CD40 differentially regulate CD22 tyrosine phosphorylation. J. Immunol. 176, 873–879 (2006).
Hokazono, Y. et al. Inhibitory coreceptors activated by antigens but not by anti-Ig heavy chain antibodies install requirement of costimulation through CD40 for survival and proliferation of B cells. J. Immunol. 171, 1835–1843 (2003).
Wakabayashi, C., Adachi, T., Wienands, J. & Tsubata, T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298, 2392–2395 (2002).
Poe, J. C. et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nature Immunol. 5, 1078–1087 (2004). This study examines the phenotype of knock-in mice bearing a mutated form of CD22 that is unable to bind sialic acids.
Ghosh, S., Bandulet, C. & Nitschke, L. Regulation of B cell development and B cell signalling by CD22 and its ligands α2,6-linked sialic acids. Int. Immunol. 18, 603–611 (2006).
Stoddart, A. et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462 (2002).
Stoddart, A., Jackson, A. P. & Brodsky, F. M. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol. Biol. Cell 16, 2339–2348 (2005).
Samardzic, T. et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 32, 561–567 (2002).
Erickson-Miller, C. L. et al. Characterization of Siglec-5 (CD170) expression and functional activity of anti-Siglec-5 antibodies on human phagocytes. Exp. Hematol. 31, 382–388 (2003).
Nguyen, D. H., Ball, E. D. & Varki, A. Myeloid precursors and acute myeloid leukemia cells express multiple CD33-related Siglecs. Exp. Hematol. 34, 728–735 (2006).
Biedermann, B., Gil, D., Bowen, D. T. & Crocker, P. R. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk. Res. 31, 211–220 (2007).
Vitale, C. et al. Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc. Natl Acad. Sci. USA 96, 15091–15096 (1999).
Balaian, L., Zhong, R. K. & Ball, E. D. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp. Hematol. 31, 363–371 (2003).
Nutku, E., Aizawa, H., Hudson, S. A. & Bochner, B. S. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101, 5014–5020 (2003).
von Gunten, S. et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106, 1423–1431 (2005). This paper and reference 94 are the first to show that ligation of Siglecs on granulocytes can trigger cell-death signals that are enhanced in the presence of cytokines that normally promote cell survival.
Paul, S. P., Taylor, L. S., Stansbury, E. K. & McVicar, D. W. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 96, 483–490 (2000).
Ulyanova, T., Shah, D. D. & Thomas, M. L. Molecular cloning of MIS, a myeloid inhibitory siglec, that binds protein-tyrosine phosphatases SHP-1 and SHP-2. J. Biol. Chem. 276, 14451–14458 (2001).
Avril, T., Floyd, H., Lopez, F., Vivier, E. & Crocker, P. R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and-9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173, 6841–6849 (2004).
Ikehara, Y., Ikehara, S. K. & Paulson, J. C. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 279, 43117–43125 (2004).
Avril, T., Freeman, S. D., Attrill, H., Clarke, R. G. & Crocker, P. R. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 280, 19843–19851 (2005).
Lajaunias, F., Dayer, J. M. & Chizzolini, C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 35, 243–251 (2005).
Orr, S. J. et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood 109, 1061–1068 (2007).
Orr, S. J. et al. SOCS3 targets Siglec 7 for proteasomal degradation and blocks Siglec 7-mediated responses. J. Biol. Chem. 282, 3418–3422 (2006).
von Gunten, S. & Simon, H. U. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 20, 601–605 (2006).
Nguyen, D. H., Hurtado-Ziola, N., Gagneux, P. & Varki, A. Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl Acad. Sci. USA 103, 7765–7770 (2006).
Zhang, J. Q., Biedermann, B., Nitschke, L. & Crocker, P. R. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur. J. Immunol. 34, 1175–1184 (2004).
Zhang, M. et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 1 Feb 2007 [epub ahead of print]. First demonstration of an inhibitory function for a CD33-related Siglec.
Lock, K., Zhang, J., Lu, J., Lee, S. H. & Crocker, P. R. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology 209, 199–207 (2004).
Zhang, J. et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 107, 3600–3608 (2006).
Walter, R. B., Raden, B. W., Kamikura, D. M., Cooper, J. A. & Bernstein, I. D. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 105, 1295–1302 (2005).
Patel, N. et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J. Biol. Chem. 274, 22729–22738 (1999).
Veillette, A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature Rev. Immunol. 6, 56–66 (2006).
Taylor, V. C. et al. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J. Biol. Chem. 274, 11505–11512 (1999). This paper establishes that a CD33-related Siglec can recruit the protein tyrosine phosphatases SHP1 and SHP2 to their ITIM and ITIM-like motif.
Yu, Z., Maoui, M., Wu, L., Banville, D. & Shen, S. mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. J. 353, 483–492 (2001).
Wong, P. K. et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J. Clin. Invest. 116, 1571–1581 (2006).
Yrlid, U. et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Crocker, P. R. et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology 8, v–vi (1998).
Blasius, A. L. & Colonna, M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 27, 255–260 (2006).
Tomasello, E. & Vivier, E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur. J. Immunol. 35, 1670–1677 (2005).
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002).
Smith, H. R. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl Acad. Sci. USA 99, 8826–8831 (2002).
Altheide, T. K. et al. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: Evidence for two modes of rapid evolution. J. Biol. Chem. 281, 25689–25702 (2006).
Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature (in the press).
Jones, G. et al. Polymorphisms within the CTLA4 gene are associated with infant atopic dermatitis. Br. J. Dermatol. 154, 467–471 (2006).
Kuroki, K. et al. Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA-DRB1 shared epitope negative rheumatoid arthritis. Hum. Mol. Genet. 14, 2469–2480 (2005).
Lin, S. C., Kuo, C. C. & Chan, C. H. Association of a BTLA gene polymorphism with the risk of rheumatoid arthritis. J. Biomed. Sci. 13, 853–860 (2006).
Tsuchiya, N., Honda, Z. & Tokunaga, K. Role of B cell inhibitory receptor polymorphisms in systemic lupus erythematosus: a negative times a negative makes a positive. J. Hum. Genet. 51, 741–750 (2006).
Crocker, P. R. et al. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 10, 1661–1669 (1991).
Acknowledgements
Work in the authors' laboratories is supported by grants from the Wellcome Trust (P.R.C.) and the National Institutes of Health, USA (J.P. and A.V.). We are grateful to T. Angata for communicating details of Siglec-15 ahead of publication and to H. Attrill for assistance in preparing Figures 2c and 2d.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- V-set immunoglobulin domain
-
A protein domain that shows evolutionary similarity, in both linear sequence and folded structure, to the variable region domains of immunoglobulins. The domain folds into a sandwich of two β-pleated sheets consisting of antiparallel β-strands. V-set domains differ from immunoglobulin constant-region type 2 (C2)-set domains by having more β-strands in the β-pleated sheets.
- C2-set immunoglobulin domain
-
A protein domain that shows evolutionary similarity, in both linear sequence and folded structure, to the immunoglobulin constant region type 2 (C2) domains. The domain folds into a sandwich of two β-pleated sheets consisting of anti-parallel β-strands. C2-set immunoglobulin domains differ from domains of the variable region of immunoglobulins by having fewer β-strands in the β-pleated sheets.
- Orthologue
-
Orthology describes genes in different species that derive from a common ancestor. Orthologous genes may or may not have the same function.
- The Red Queen effect
-
A term that describes unremitting evolutionary arms races that can occur between competing species, or between a pathogen and its host. The term is derived from the Red Queen's comment in Lewis Carroll's Through the Looking Glass: “It takes all the running you can do, to keep in the same place.”
- Immunoreceptor tyrosine-based inhibitory motif
-
(ITIM). A short peptide motif containing a tyrosine residue that is found in the cytoplasmic regions of many inhibitory receptors. The consensus sequence is (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val), with X denoting any amino acid. Following tyrosine phosphorylation by SRC-family protein tyrosine kinases, this provides a high-affinity docking site for the recruitment of cytoplasmic phosphatases and other signalling molecules with an appropriate SRC homology 2 (SH2) domain.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A short peptide motif containing tyrosine residues that is found in the cytoplasmic tails of several signalling molecules and in adaptors such as DAP12. The consensus sequence is (Asp/Glu)-X-X-Tyr-X-X-(Leu/Ile)-X6–8-Tyr-X-X-(Leu/Ile), with X denoting any amino acid. It is tyrosine phosphorylated after engagement of the ligand-binding subunits, which triggers a cascade of intracellular events that usually results in cellular activation.
- Glycome
-
The entire set of glycans in a cell, tissue or organism, under specified conditions. The sizes of glycomes are currently unknown but are likely to be many-fold larger than the size of the corresponding proteome owing to the combinatorial complexity and dynamic variability of glycan structures.
- Counter-receptor
-
A term used to describe the combination of oligosaccharide ligands coupled to protein or lipid carriers47. For many glycan-binding proteins, the affinity for oligosaccharide ligands is low, but high-avidity multivalent binding can occur when the ligands are appropriately displayed on carriers.
- Clathrin domains
-
Specialized membrane microdomains that mediate endocytosis to early endosomes by a mechanism involving the formation of clathrin cages on the cytoplasmic face of the plasma membrane.
- Proliferative glomerulonephritis
-
A group of inflammatory diseases affecting the glomerular apparatus of the kidney. These diseases have varied aetiologies but characteristically exhibit proliferation of mesangial cells and endocapillaries, and infiltration of leukocytes, such as macrophages and T cells.
- Experimental autoimmune uveoretinitis
-
A photoreceptor-specific autoimmune disease that is inducible in several susceptible animal models with various retinal autoantigens. It resembles some human posterior uveoretinitis syndromes, including sympathetic ophthalmia, Vogt–Koyanagi–Harada disease, sarcoidosis, Behçet's disease and birdshot retinochoroidopathy.
- Mixed lymphocyte reactions
-
A tissue-culture technique that is used for the in vitro testing of the proliferative response of T cells from one individual to lymphocytes from another individual.
- LYN-deficient mouse model of lupus
-
A deficiency of the phosphokinase LYN results in a hyperimmune status leading to an autoimmune condition that is similar to the human disease systemic lupus.
- Rafts or activation rafts
-
Membrane microdomains enriched in glycosphingolipids, where cell signalling receptors form macromolecular complexes with other proteins involved in the initiation of cell-activation pathways.
- Paralogue
-
Paralogy describes homologous genes in a single species that diverged by gene duplication. Paralogues are more likely to evolve new functions. Siglec-F and Siglec-8 are unusual in that they are paralogues that have developed similarities in cell-type expression and binding specificity by convergent evolution.
- Suppressors of cytokine signalling 3
-
(SOCS 3). A member of the family of eight cytoplasmic proteins (SOCS1–SOCS7 and CIS) that contain an amino-terminal region of variable length, a central SH2 domain and a carboxy-terminal SOCS box. SOCS proteins provide a negative-feedback loop to attenuate signal transduction from cytokine receptors that act through the JAK–STAT (Janus kinase –signal transducer and activator of transcription) pathway.
- Cross-presentation
-
The initiation of a CD8+ T-cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC-class-I pathway of antigen presentation.
- Paired receptors
-
These are membrane proteins, one of which is potentially inhibitory and the other activating and which are highly related to each other in the extracellular domain but differ significantly in the transmembrane and cytoplasmic regions.
Rights and permissions
About this article
Cite this article
Crocker, P., Paulson, J. & Varki, A. Siglecs and their roles in the immune system. Nat Rev Immunol 7, 255–266 (2007). https://doi.org/10.1038/nri2056
Issue Date:
DOI: https://doi.org/10.1038/nri2056
This article is cited by
-
Identification of therapeutic targets and prognostic biomarkers in the Siglec family of genes in tumor immune microenvironment of sarcoma
Scientific Reports (2024)
-
The Contribution of Serum Sialic Acid Binding Immunoglobulin-Like Lectin 1(sSIGLEC-1) as an IFN I Signature Biomarker in the Progression of Atherosclerosis in Egyptian Systemic Lupus Erythematosus (SLE) Patients
Indian Journal of Clinical Biochemistry (2024)
-
CD33 as a leukocyte-associated marker expressed on human spermatozoa
BMC Research Notes (2023)
-
Polymeric biomaterial-inspired cell surface modulation for the development of novel anticancer therapeutics
Biomaterials Research (2023)
-
The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells
Nature (2023)