Key Points
-
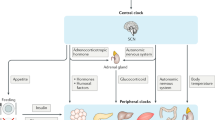
Circadian rhythms are endogenously generated rhythms that occur with a periodicity of around 24 hours.
-
There is an intimate link between the disruption of circadian rhythms and cancer. The circadian clock regulates key aspects of cell growth and survival, including the cell cycle, DNA damage responses, cellular senescence and metabolism.
-
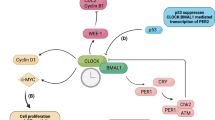
Several cell cycle genes, such as WEE1, MYC and cyclin D1, are regulated by the circadian clock. This could be one way by which the circadian clock gates cell division.
-
Key clock proteins, such as PER1 and timeless, interact with proteins involved in the DNA damage responses. DNA damage itself can reset the clock.
-
Cellular metabolism is altered in cancer. Several key metabolic genes are under circadian regulation. Recent findings that SIRT1, a key regulator of metabolism, is an integral part of the clock machinery suggest that the circadian clock can regulate cellular metabolism in multiple ways.
-
The transcriptional negative feedback loop that regulates the circadian clock is interlocked with an enzymatic feedback loop in which SIRT1 regulates the levels of its own cofactor, NAD+.
Abstract
Circadian rhythms govern a remarkable variety of metabolic and physiological functions. Accumulating epidemiological and genetic evidence indicates that the disruption of circadian rhythms might be directly linked to cancer. Intriguingly, several molecular gears constituting the clock machinery have been found to establish functional interplays with regulators of the cell cycle, and alterations in clock function could lead to aberrant cellular proliferation. In addition, connections between the circadian clock and cellular metabolism have been identified that are regulated by chromatin remodelling. This suggests that abnormal metabolism in cancer could also be a consequence of a disrupted circadian clock. Therefore, a comprehensive understanding of the molecular links that connect the circadian clock to the cell cycle and metabolism could provide therapeutic benefit against certain human neoplasias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Compagni, A. & Christofori, G. Recent advances in research on multistage tumorigenesis. Br. J. Cancer 83, 1–5 (2000).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 23, 537–548 (2009).
Sahar, S. & Sassone-Corsi, P. Circadian clock and breast cancer: a molecular link. Cell Cycle 6, 1329–1331 (2007).
Matsuo, T. et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259 (2003).
Green, C. B., Takahashi, J. S. & Bass, J. The meter of metabolism. Cell 134, 728–742 (2008).
Schibler, U. & Sassone-Corsi, P. A web of circadian pacemakers. Cell 111, 919–922 (2002).
Merrow, M., Spoelstra, K. & Roenneberg, T. The circadian cycle: daily rhythms from behaviour to genes. EMBO Rep. 6, 930–935 (2005).
Stevens, R. G. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 16, 254–258 (2005).
Schernhammer, E. S. et al. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J. Natl Cancer Inst. 93, 1563–1568 (2001).
Hansen, J. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12, 74–77 (2001).
Schernhammer, E. S. et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 100, 898–905 (2008).
Travis, R. C., Allen, D. S., Fentiman, I. S. & Key, T. J. Melatonin and breast cancer: a prospective study. J. Natl Cancer Inst. 96, 475–482 (2004).
Lis, C. G. et al. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integr Cancer Ther. 2, 105–111 (2003).
Levi, F. et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv. Drug Deliv. Rev. 59, 1015–1035 (2007).
Kobayashi, M., Wood, P. A. & Hrushesky, W. J. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 19, 237–251 (2002).
Glossop, N. R. & Hardin, P. E. Central and peripheral circadian oscillator mechanisms in flies and mammals. J. Cell Sci. 115, 3369–3377 (2002).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Tsubouchi, H., Kamibeppu, A., Fujisaki, K., Nagahama, J. & Hashimoto, S. Hepatic gluconeogenic key enzymes in patients with hepatic cancer. Gastroenterol. Jpn 15, 564–569 (1980).
Young, M. W. & Kay, S. A. Time zones: a comparative genetics of circadian clocks. Nature Rev. Genet. 2, 702–715 (2001).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002).
Hastings, M. H., Reddy, A. B. & Maywood, E. S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Rev. Neurosci. 4, 649–661 (2003).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009).
Ramsey, K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009). This paper and reference 23 demonstrate that the metabolite NAD+ oscillates and is controlled by the circadian clock. There are many conceptual implications, including that SIRT1 directs the intracellular levels of its own cofactor.
Shearman, L. P., Zylka, M. J., Weaver, D. R., Kolakowski, L. F. Jr & Reppert, S. M. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269 (1997).
Albrecht, U., Sun, Z. S., Eichele, G. & Lee, C. C. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91, 1055–1064 (1997).
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998).
Akashi, M. & Nishida, E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev. 14, 645–649 (2000).
Sanada, K., Okano, T. & Fukada, Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J. Biol. Chem. 277, 267–271 (2002).
Shim, H. S. et al. Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO Rep. 8, 366–371 (2007).
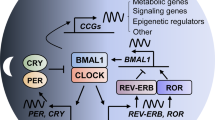
Gallego, M. & Virshup, D. M. Post-translational modifications regulate the ticking of the circadian clock. Nature Rev. Mol. Cell Biol. 8, 139–148 (2007).
Kaasik, K. & Lee, C. C. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430, 467–471 (2004).
Sato, T. K. et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43, 527–537 (2004).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Reick, M., Garcia, J. A., Dudley, C. & McKnight, S. L. NPAS2: an analog of clock operative in the mammalian forebrain. Science 293, 506–509 (2001).
DeBruyne, J. P., Weaver, D. R. & Reppert, S. M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nature Neurosci. 10, 543–545 (2007).
DeBruyne, J. P., Weaver, D. R. & Reppert, S. M. Peripheral circadian oscillators require CLOCK. Curr. Biol. 17, R538–539 (2007). A report that clarifies that the role of CLOCK is prominent in peripheral tissues, rather than in the central pacemaker in the SCN.
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005).
Kondratov, R. V. et al. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle 5, 890–895 (2006).
Dardente, H., Fortier, E. E., Martineau, V. & Cermakian, N. Cryptochromes impair phosphorylation of transcriptional activators in the clock: a general mechanism for circadian repression. Biochem. J. 402, 525–536 (2007).
Shirogane, T., Jin, J., Ang, X. L. & Harper, J. W. SCFβ-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J. Biol. Chem. 280, 26863–26872 (2005).
Akashi, M., Tsuchiya, Y., Yoshino, T. & Nishida, E. Control of intracellular dynamics of mammalian period proteins by casein kinase I ɛ (CKIɛ) and CKIδ in cultured cells. Mol. Cell Biol. 22, 1693–1703 (2002).
Busino, L. et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316, 900–904 (2007).
Godinho, S. I. et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900 (2007).
Siepka, S. M. et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129, 1011–1023 (2007). This report and references 43 and 44 reveal the first example of a circadian-specific F-box protein that controls the stability of clock regulators.
Cardone, L. et al. Circadian clock control by SUMOylation of BMAL1. Science 309, 1390–1394 (2005).
Hirayama, J. et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450, 1086–1090 (2007).
Etchegaray, J. P., Lee, C., Wade, P. A. & Reppert, S. M. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 (2003).
Ripperger, J. A. & Schibler, U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nature Genet. 38, 369–374 (2006).
Doi, M., Hirayama, J. & Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508 (2006).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
Asher, G. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008). This study and reference 51 first linked SIRT1 to the clock machinery. SIRT1 is a regulator of metabolism, ageing and inflammation.
Chen, S. T. et al. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 26, 1241–1246 (2005).
Hoffman, A. E., Zheng, T., Ba, Y. & Zhu, Y. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Mol. Cancer Res. 6, 1461–1468 (2008).
Gery, S. et al. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382 (2006).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002).
Gery, S. et al. Transcription profiling of C/EBP targets identifies Per2 as a gene implicated in myeloid leukemia. Blood 106, 2827–2836 (2005).
Yang, W. S. & Stockwell, B. R. Inhibition of casein kinase 1-epsilon induces cancer-cell-selective, PERIOD2-dependent growth arrest. Genome Biol. 9, R92 (2008).
Cermakian, N., Monaco, L., Pando, M. P., Dierich, A. & Sassone-Corsi, P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 20, 3967–3974 (2001).
Zheng, B. et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 (2001).
Bae, K. et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30, 525–536 (2001).
Zheng, B. et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400, 169–173 (1999).
Gery, S., Virk, R. K., Chumakov, K., Yu, A. & Koeffler, H. P. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 26, 7916–7920 (2007).
Green, K. A. & Carroll, J. S. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nature Rev. Cancer 7, 713–722 (2007).
Hirayama, J. & Sassone-Corsi, P. Structural and functional features of transcription factors controlling the circadian clock. Curr. Opin. Genet. Dev. 15, 548–556 (2005).
Zylka, M. J. et al. Molecular analysis of mammalian timeless. Neuron 21, 1115–1122 (1998).
Gauger, M. A. & Sancar, A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 65, 6828–6834 (2005).
Antoch, M. P. et al. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 7, 1197–1204 (2008).
Miller, B. H. et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl Acad. Sci. USA 104, 3342–3347 (2007).
Ozturk, N., Lee, J. H., Gaddameedhi, S. & Sancar, A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl Acad. Sci. USA (2009). A study that establishes a solid link between the Cry circadian regulators and the p53 tumour suppressor.
Hunt, T. & Sassone-Corsi, P. Riding tandem: circadian clocks and the cell cycle. Cell 129, 461–464 (2007).
Canaple, L., Kakizawa, T. & Laudet, V. The days and nights of cancer cells. Cancer Res. 63, 7545–7552 (2003).
Rensing, L. & Goedeke, K. Circadian rhythm and cell cycle: possible entraining mechanisms. Chronobiologia 3, 853–865 (1976).
Hirayama, J., Cardone, L., Doi, M. & Sassone-Corsi, P. Common pathways in circadian and cell cycle clocks: light-dependent activation of Fos/AP-1 in zebrafish controls CRY-1a and WEE-1. Proc. Natl Acad. Sci. USA 102, 10194–10199 (2005).
Roy, P. G. & Thompson, A. M. Cyclin D1 and breast cancer. Breast 15, 718–727 (2006).
Kang, T. H., Reardon, J. T., Kemp, M. & Sancar, A. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc. Natl Acad. Sci. USA (2009).
Barnes, J. W. et al. Requirement of mammalian Timeless for circadian rhythmicity. Science 302, 439–442 (2003).
Unsal-Kacmaz, K., Mullen, T. E., Kaufmann, W. K. & Sancar, A. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell Biol. 25, 3109–3116 (2005).
Oklejewicz, M. et al. Phase resetting of the mammalian circadian clock by DNA damage. Curr. Biol. 18, 286–291 (2008).
Gamsby, J. J., Loros, J. J. & Dunlap, J. C. A phylogenetically conserved DNA damage response resets the circadian clock. J. Biol. Rhythms 24, 193–202 (2009).
Pregueiro, A. M., Liu, Q., Baker, C. L., Dunlap, J. C. & Loros, J. J. The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313, 644–649 (2006).
Klevecz, R. R., Bolen, J., Forrest, G. & Murray, D. B. A genomewide oscillation in transcription gates DNA replication and cell cycle. Proc. Natl Acad. Sci. USA 101, 1200–1205 (2004).
Chen, Z., Odstrcil, E. A., Tu, B. P. & McKnight, S. L. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science 316, 1916–1919 (2007).
Eckel-Mahan, K. & Sassone-Corsi, P. Metabolism control by the circadian clock and vice versa. Nature Struct. Mol. Biol. 16, 462–467 (2009).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
Green, C. B. et al. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc. Natl Acad. Sci. USA 104, 9888–9893 (2007).
Yang, X. et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 126, 801–810 (2006).
Liu, C., Li, S., Liu, T., Borjigin, J. & Lin, J. D. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447, 477–481 (2007). A study that links the clock to a regulator of metabolism, the function of which in inflammation and cancer is also described.
Oishi, K. et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J. Biol. Chem. 278, 41519–41527 (2003).
Alenghat, T. et al. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456, 997–1000 (2008).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Brooks, C. L. & Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nature Rev. Cancer 9, 123–128 (2009).
Vaziri, H. et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Chen, W. Y. et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 123, 437–448 (2005).
Cohen, H. Y. et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell 13, 627–638 (2004).
Wang, R. H. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312–323 (2008).
Oberdoerffer, P. et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918 (2008).
Firestein, R. et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 3, e2020 (2008).
Schwer, B. & Verdin, E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 7, 104–112 (2008).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Li, X. et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 28, 91–106 (2007).
Crosio, C., Cermakian, N., Allis, C. D. & Sassone-Corsi, P. Light induces chromatin modification in cells of the mammalian circadian clock. Nature Neurosci. 3, 1241–1247 (2000).
Borrelli, E., Nestler, E. J., Allis, C. D. & Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974 (2008).
Kolthur-Seetharam, U., Dantzer, F., McBurney, M. W., de Murcia, G. & Sassone-Corsi, P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 5, 873–877 (2006).
Dantzer, F. et al. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 409, 493–510 (2006).
Pillai, J. B., Isbatan, A., Imai, S. & Gupta, M. P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J. Biol. Chem. 280, 43121–43130 (2005).
Martin, S. A., Lord, C. J. & Ashworth, A. DNA repair deficiency as a therapeutic target in cancer. Curr. Opin. Genet. Dev. 18, 80–86 (2008).
Garten, A., Petzold, S., Korner, A., Imai, S. I. & Kiess, W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 20, 130–138 (2008).
Hasmann, M. & Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 63, 7436–7442 (2003).
Demierre, M. F., Higgins, P. D., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nature Rev. Cancer 5, 930–942 (2005).
Brown, M. S., Goldstein, J. L. & Dietschy, J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J. Biol. Chem. 254, 5144–5149 (1979).
Klevecz, R. R., Shymko, R. M., Blumenfeld, D. & Braly, P. S. Circadian gating of S phase in human ovarian cancer. Cancer Res. 47, 6267–6271 (1987).
Smaaland, R., Lote, K., Sothern, R. B. & Laerum, O. D. DNA synthesis and ploidy in non-Hodgkin's lymphomas demonstrate intrapatient variation depending on circadian stage of cell sampling. Cancer Res. 53, 3129–3138 (1993).
Gorbacheva, V. Y. et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc. Natl Acad. Sci. USA 102, 3407–3412 (2005).
DePinho, R. A. The age of cancer. Nature 408, 248–254 (2000).
Kondratov, R. V. A role of the circadian system and circadian proteins in aging. Ageing Res. Rev. 6, 12–27 (2007).
Hofman, M. A. & Swaab, D. F. Living by the clock: the circadian pacemaker in older people. Ageing Res. Rev. 5, 33–51 (2006).
Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 20, 1868–1873 (2006). Mice deficient in the circadian regulator BMAL1 have a phenotype reminiscent of animals lacking SIRT1.
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Vitaterna, M. H. et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725 (1994).
King, D. P. et al. Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 (1997).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
Debruyne, J. P. et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50, 465–477 (2006).
Haigis, M. C. & Guarente, L. P. Mammalian sirtuins — emerging roles in physiology, aging, and calorie restriction. Genes Dev. 20, 2913–2921 (2006).
Kim, D. et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179 (2007).
Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 (2004).
Lamia, K. A., Storch, K. F. & Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl Acad. Sci. USA 105, 15172–15177 (2008).
Vitaterna, M. H. et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl Acad. Sci. USA 96, 12114–12119 (1999).
Thresher, R. J. et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998).
van der Horst, G. T. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999).
Dudley, C. A. et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301, 379–383 (2003).
Meng, Q. J. et al. Setting clock speed in mammals: the CK1ɛ tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58, 78–88 (2008).
Lowrey, P. L. et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–492 (2000).
Etchegaray, J. P. et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol. 29, 3853–3866 (2009).
Raspe, E. et al. Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J. Lipid Res. 43, 2172–2179 (2002).
Mamontova, A. et al. Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORα. Circulation 98, 2738–2743 (1998).
Lin, J. et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119, 121–135 (2004).
Leone, T. C. et al. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101 (2005).
Gumz, M. L. et al. The circadian clock protein period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Invest. 119, 2423–2434 (2009).
Acknowledgements
We wish to apologize to all colleagues whose work, because of lack of space, could not be cited. We thank all the members of the Sassone-Corsi laboratory for helpful discussions. S.S. is supported by a postdoctoral fellowship from the American Heart Association, Western States Affiliates, USA. Work in our laboratory is supported by the National Institute of Health, USA, and the Institut de la Sante et de la Recherche Medicale, France.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Zeitgeber
-
A German word that literally means 'time giver', it refers to external cues that entrain the endogenous clock.
- Melatonin
-
A hormone, primarily synthesized by the pineal gland, which has highest circulating levels at night.
- E-box
-
A stretch of DNA with the sequence CACGTG, which is the most common motif in the mammalian genome.
- Ultradian rhythms
-
Rhythms with a periodicity of less than 24 hours that are repeated several times in a circadian cycle. For example, heart rate and yeast metabolic rhythms.
- Gluconeogenesis
-
The synthesis of glucose from non-carbohydrate sources (such as lactate and amino acids).
- Nucleotide excision repair
-
A DNA repair process in which a short single-stranded region encompassing a DNA mutation is replaced. Defects in NER can lead to diseases such as Xeroderma pigmentosum.
- Metabolic syndrome
-
A collection of risk factors, including high blood sugar levels, high blood pressure, large waistline, high blood triglyceride levels and low HDL blood cholesterol, which increases the risk for type 2 diabetes and cardiovascular diseases.
- Hyperglycaemia
-
A condition in which blood glucose levels are high. It is generally a consequence either of low levels of insulin or insulin resistance.
- Dyslipidemia
-
A risk factor for cardiovascular disease that refers to higher levels of triglycerides and low-density lipoprotein cholesterol but lower levels of HDL cholesterol in blood.
- Hepatic steatosis
-
A condition, also known as fatty liver, in which large droplets of triglycerides accumulate in liver cells. It is commonly associated with alcoholism and obesity.
Rights and permissions
About this article
Cite this article
Sahar, S., Sassone-Corsi, P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer 9, 886–896 (2009). https://doi.org/10.1038/nrc2747
Issue Date:
DOI: https://doi.org/10.1038/nrc2747
This article is cited by
-
Differential expression of the circadian clock network correlates with tumour progression in gliomas
BMC Medical Genomics (2023)
-
Construction and verification of a novel circadian clock related long non-coding RNA model and prediction of treatment for survival prognosis in patients with hepatocellular carcinoma
BMC Cancer (2023)
-
SCFβTrCP-mediated degradation of SHARP1 in triple-negative breast cancer
Cell Death & Disease (2023)
-
Nucleus-exported CLOCK acetylates PRPS to promote de novo nucleotide synthesis and liver tumour growth
Nature Cell Biology (2023)
-
Circadian disruption alters gut barrier integrity via a ß-catenin-MMP-related pathway
Molecular and Cellular Biochemistry (2023)