Abstract
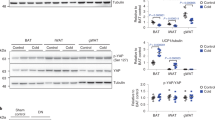
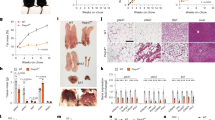
The adipocyte-derived secretory factor adiponectin promotes insulin sensitivity, decreases inflammation and promotes cell survival. No unifying mechanism has yet explained how adiponectin can exert such a variety of beneficial systemic effects. Here, we show that adiponectin potently stimulates a ceramidase activity associated with its two receptors, AdipoR1 and AdipoR2, and enhances ceramide catabolism and formation of its antiapoptotic metabolite—sphingosine-1-phosphate (S1P)—independently of AMP-dependent kinase (AMPK). Using models of inducible apoptosis in pancreatic beta cells and cardiomyocytes, we show that transgenic overproduction of adiponectin decreases caspase-8-mediated death, whereas genetic ablation of adiponectin enhances apoptosis in vivo through a sphingolipid-mediated pathway. Ceramidase activity is impaired in cells lacking both adiponectin receptor isoforms, leading to elevated ceramide levels and enhanced susceptibility to palmitate-induced cell death. Combined, our observations suggest a unifying mechanism of action for the beneficial systemic effects exerted by adiponectin, with sphingolipid metabolism as its core upstream signaling component.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yamauchi, T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003).
Combs, T.P. et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145, 367–383 (2004).
Kim, J.Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Nawrocki, A.R. et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281, 2654–2660 (2006).
Kubota, N. et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 277, 25863–25866 (2002).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Yamauchi, T. et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 (2007).
Iwabu, M. et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464, 1313–1319 (2010).
Schraw, T., Wang, Z.V., Halberg, N., Hawkins, M. & Scherer, P.E. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149, 2270–2282 (2008).
Halberg, N. et al. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58, 1961–1970 (2009).
Merrill, A.H. Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 (2002).
Holland, W.L. & Summers, S.A. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29, 381–402 (2008).
Savage, D.B., Petersen, K.F. & Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87, 507–520 (2007).
Kim, J.K. et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Invest. 114, 823–827 (2004).
Yu, C. et al. Mechanism by which fatty acids inhibit insulin activation of IRS-1 associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 (2002).
Benoit, S.C. et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J. Clin. Invest. 119, 2577–2589 (2009).
Stratford, S., Hoehn, K.L., Liu, F. & Summers, S.A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 (2004).
Mukhopadhyay, A. et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 23, 751–763 (2009).
Bourbon, N.A., Yun, J., Berkey, D., Wang, Y. & Kester, M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am. J. Physiol. Cell Physiol. 280, C1403–C1411 (2001).
Powell, D.J., Hajduch, E., Kular, G. & Hundal, H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 23, 7794–7808 (2003).
Fox, T.E. et al. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J. Biol. Chem. 282, 12450–12457 (2007).
Takabe, K., Paugh, S.W., Milstien, S. & Spiegel, S. ″Inside-out″ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60, 181–195 (2008).
Combs, T.P., Berg, A.H., Obici, S., Scherer, P.E. & Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875–1881 (2001).
Berg, A.H., Combs, T.P., Du, X., Brownlee, M. & Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001).
Pajvani, U.B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11, 797–803 (2005).
Wang, Z.V. et al. PANIC-ATTAC: a mouse model for inducible and reversible beta-cell ablation. Diabetes 57, 2137–2148 (2008).
Wencker, D. et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 111, 1497–1504 (2003).
Brakch, N. et al. Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur. Heart J. 31, 67–76 (2010).
Gleason, C.E., Lu, D., Witters, L.A., Newgard, C.B. & Birnbaum, M.J. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J. Biol. Chem. 282, 10341–10351 (2007).
Mao, C. & Obeid, L.M. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta 1781, 424–434 (2008).
Villa, N.Y. et al. Sphingolipids function as downstream effectors of a fungal PAQR. Mol. Pharmacol. 75, 866–875 (2009).
Shaw, R.J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 101, 3329–3335 (2004).
Holland, W.L. & Scherer, P.E. PAQRs: a counteracting force to ceramides? Mol. Pharmacol. 75, 740–743 (2009).
Aerts, J.M. et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56, 1341–1349 (2007).
Holland, W.L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Yamashita, T. et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. USA 100, 3445–3449 (2003).
Zhao, H. et al. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56, 1210–1218 (2007).
El Bawab, S. et al. Substrate specificity of rat brain ceramidase. J. Lipid Res. 43, 141–148 (2002).
Asterholm, I.W. & Scherer, P.E. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J. Pathol. 176, 1364–1376 (2010).
Puigserver, P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int. J. Obes. (Lond.) 29 (suppl. 1), S5–S9 (2005).
Levine, Y.C., Li, G.K. & Michel, T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK → Rac1 → Akt → endothelial nitric-oxide synthase pathway. J. Biol. Chem. 282, 20351–20364 (2007).
Olivera, A. et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26, 287–297 (2007).
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 (2003).
Hardie, D.G. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. (Lond.) 32 (suppl. 4), S7–S12 (2008).
Karliner, J.S. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J. Cardiovasc. Pharmacol. 53, 189–197 (2009).
Van Veldhoven, P.P., Gijsbers, S., Mannaerts, G.P., Vermeesch, J.R. & Brys, V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim. Biophys. Acta 1487, 128–134 (2000).
Kupchak, B.R. et al. Probing the mechanism of FET3 repression by Izh2p overexpression. Biochim. Biophys. Acta 1773, 1124–1132 (2007).
King, C.C. et al. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 275, 18108–18113 (2000).
Clackson, T. et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. USA 95, 10437–10442 (1998).
Pajvani, U.B. et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J. Biol. Chem. 278, 9073–9085 (2003).
Li, Z. et al. Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J. Biol. Chem. 284, 27010–27019 (2009).
Perry, D.K., Bielawska, A. & Hannun, Y.A. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol. 312, 22–31 (2000).
Berglund, E.D. et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093 (2009).
Stein, D.T. et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 100, 398–403 (1997).
Mao, C. et al. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J. Biol. Chem. 276, 26577–26588 (2001).
Mu, J., Brozinick, J.T. Jr. Valladares, O., Bucan, M. & Birnbaum, M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 (2001).
Burger, D. et al. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc. Res. 72, 51–59 (2006).
Hohmeier, H.E. et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 (2000).
Hohmeier, H.E., Tran, V.V., Chen, G., Gasa, R. & Newgard, C.B. Inflammatory mechanisms in diabetes: lessons from the beta-cell. Int. J. Obes. Relat. Metab. Disord. 27 (suppl. 3), S12–S16 (2003).
He, L. & Fox, M.H. Variation of heat shock protein 70 through the cell cycle in HL-60 cells and its relationship to apoptosis. Exp. Cell Res. 232, 64–71 (1997).
Acknowledgements
We thank members of the Scherer and Summers laboratories for comments. We would like to thank R. Kitsis for discussions regarding the generation of HEART-ATTAC mice. We thank B. Hammer and the Transgenic Core Facility at UT Southwestern for the generation of the mouse models used in this study and the Metabolic Core Facility at UT Southwestern for help with phenotyping of the mice. Lkb1−/− mice and cells were a kind gift from R. DePinho, Massachusetts General Hospital. INS-1 832/13 cells were generously provided by C. Newgard, Duke University Medical Center. We thank Ariad Pharmaceuticals for providing the dimerization kit and compound AP20187. This work was supported by US National Institutes of Health grants R01-DK55758, R01-CA112023, RC1-DK086629 and P01-DK088761 (P.E.S.); R01-DK56886 and P01-DK49210 (M.J.B.); as well as R21-DK073181 (S.A.S.). W.L.H. was supported by National Research Service Award F32-DK083866 and TL1-DK081181. J.M.R. was supported by F32-DK085935 and T32-HL007360 and K.E.D. was supported by F32-DK081279. N.H. was funded by a grant from University of Copenhagen.
Author information
Authors and Affiliations
Contributions
W.L.H. conducted all experiments, except the portions indicated below, and contributed to the writing of the manuscript. R.A.M. conducted in vivo experiments with liver-specific Lkb1−/− mice. Z.V.W. generated all of the ATTAC mouse models used here. K.S. was responsible for the mutagenesis studies of AdipoR1 and AdipoR2. B.M.B. was involved in the studies with INS-1 cells. H.H.B., M.R.W., M.-S.K. and J.T.B. were involved in liquid chromatography–tandem mass spectrometry analysis for determination of sphingolipid content of the samples. K.E.D. assisted in the generation of the Adipor1−/−Adipor2−/− MEFs and high-fat feeding studies using adiponectin transgenic mice. B.T.B. helped in data analysis and RT-PCR of sphingolipid metabolism genes. N.H. performed the experiments with in vivo injections of adiponectin and detection of the protein in beta cells. J.M.R. was involved in designing experiments and protein production. V.M.T. performed ceramidase assays and genotyping. B.B.Z., M.J.B., S.A.S. and P.E.S. were involved in experimental design, data analysis and in the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary Methods (PDF 2792 kb)
Rights and permissions
About this article
Cite this article
Holland, W., Miller, R., Wang, Z. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17, 55–63 (2011). https://doi.org/10.1038/nm.2277
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2277
This article is cited by
-
Sphingolipids: drivers of cardiac fibrosis and atrial fibrillation
Journal of Molecular Medicine (2024)
-
Effects of half-dose spiomet treatment in girls with early puberty and accelerated bone maturation: a multicenter, randomized, placebo-controlled study protocol
Trials (2023)
-
Sex differences in hearing impairment due to diet-induced obesity in CBA/Ca mice
Biology of Sex Differences (2023)
-
Potential Protective Function of Adiponectin in Diabetic Retinopathy
Ophthalmology and Therapy (2023)
-
Endogenous renal adiponectin drives gluconeogenesis through enhancing pyruvate and fatty acid utilization
Nature Communications (2023)