Abstract
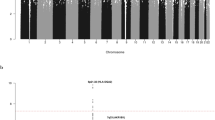
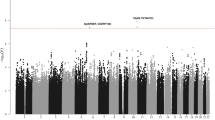
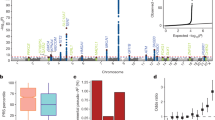
To identify risk variants for childhood acute lymphoblastic leukemia (ALL), we conducted a genome-wide association study of two case-control series, analyzing the genotypes with respect to 291,423 tagging SNPs in a total of 907 ALL cases and 2,398 controls. We identified risk loci for ALL at 7p12.2 (IKZF1, rs4132601, odds ratio (OR) = 1.69, P = 1.20 × 10−19), 10q21.2 (ARID5B, rs7089424, OR = 1.65, P = 6.69 × 10−19) and 14q11.2 (CEBPE, rs2239633, OR = 1.34, P = 2.88 × 10−7). The 10q21.2 (ARID5B) risk association appears to be selective for the subset of B-cell precursor ALL with hyperdiploidy. These data show that common low-penetrance susceptibility alleles contribute to the risk of developing childhood ALL and provide new insight into disease causation of this specific hematological cancer. Notably, all three risk variants map to genes involved in transcriptional regulation and differentiation of B-cell progenitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stiller, C.A. & Parkin, D.M. Geographic and ethnic variations in the incidence of childhood cancer. Br. Med. Bull. 52, 682–703 (1996).
Pui, C.H., Relling, M.V. & Downing, J.R. Acute lymphoblastic leukemia. N. Engl. J. Med. 350, 1535–1548 (2004).
Greaves, M. Infection, immune responses and the aetiology of childhood leukaemia. Nat. Rev. Cancer 6, 193–203 (2006).
Hemminki, K. & Jiang, Y. Risks among siblings and twins for childhood acute lymphoid leukaemia: results from the Swedish Family-Cancer Database. Leukemia 16, 297–298 (2002).
Couto, E., Chen, B. & Hemminki, K. Association of childhood acute lymphoblastic leukaemia with cancers in family members. Br. J. Cancer 93, 1307–1309 (2005).
Hodgson, S. & Maher, E. A Practical Guide to Human Cancer Genetics (Cambridge University Press, Cambridge, UK, 2007).
UK Childhood Cancer Study Investigators. The United Kingdom Childhood Cancer Study: objectives, materials and methods. Br. J. Cancer 82, 1073–1102 (2000).
Power, C. & Elliott, J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int. J. Epidemiol. 35, 34–41 (2006).
Tomlinson, I.P. et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat. Genet. 40, 623–630 (2008).
Clayton, D.G. et al. Population structure, differential bias and genomic control in a large-scale, case-control association study. Nat. Genet. 37, 1243–1246 (2005).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Harker, N. et al. The CD8α gene locus is regulated by the Ikaros family of proteins. Mol. Cell 10, 1403–1415 (2002).
Georgopoulos, K. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 (1994).
Klug, C.A. et al. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc. Natl. Acad. Sci. USA 95, 657–662 (1998).
Mullighan, C.G. et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 360, 470–480 (2009).
Mullighan, C.G. et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453, 110–114 (2008).
Wilsker, D., Patsialou, A., Dallas, P.B. & Moran, E. ARID proteins: a diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 13, 95–106 (2002).
Lahoud, M.H. et al. Gene targeting of Desrt, a novel ARID class DNA-binding protein, causes growth retardation and abnormal development of reproductive organs. Genome Res. 11, 1327–1334 (2001).
Chang, L.W. et al. Computational identification of the normal and perturbed genetic networks involved in myeloid differentiation and acute promyelocytic leukemia. Genome Biol. 9, R38 (2008).
Akasaka, T. et al. Five members of the CEBP transcription factor family are targeted by recurrent IGH translocations in B-cell precursor acute lymphoblastic leukemia (BCP-ALL). Blood 109, 3451–3461 (2007).
Pettiti, D. Meta-analysis, Decision Analysis, and Cost-effectiveness Analysis: Methods for Quantitative Synthesis in Medicine (Oxford University Press, Oxford and New York, 1994).
Higgins, J.P. & Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Stranger, B.E. et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 1, e78 (2005).
Stranger, B.E. et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315, 848–853 (2007).
Cuzick, J.A. Wilcoxon-type test for trend. Stat. Med. 4, 87–90 (1985).
Acknowledgements
Leukemia Research (UK) and the Kay Kendall Leukemia Fund provided principal funding for this study. Support from Cancer Research UK (C1298/A8362 supported by the Bobby Moore Fund) is also acknowledged. The study made use of genotyping data obtained from the British 1958 Birth Cohort. Genotyping data on 1958 controls was generated and generously supplied to us by P. Deloukas of the Wellcome Trust Sanger Institute. A full list of the investigators who contributed to the generation of the 1958 data is available from http://www.wtccc.org.uk/. We acknowledge use of DNA from the British 1958 Birth Cohort collection, funded by the UK Medical Research Council (MRC) grant G0000934 and the Wellcome Trust grant 068545/Z/02. We are grateful to S. Richards and J. Burrett (Clinical Trials Service Unit, Oxford), C. Harrison, L. Chilton and A. Moorman (Leukaemia Research Cytogenetics Group, Northern Institute for Cancer Research, Newcastle University), J. Simpson (University of York), P. Thomson and A. Hussain (Cancer Immunogenetics, School of Cancer Sciences, University of Manchester) for assistance with data harmonization, and also to I. Roberts and The Children's Cancer and Leukemia Group Biological Studies Steering Group for access to MRC ALL Trial samples. P. Thomson is funded by Children with Leukemia, and we acknowledge their support. We acknowledge UK National Health Service funding to the National Institutes for Health Research Biomedical Research Centre. Finally, we are grateful to all study participants for their participation. We also thank the clinicians, other hospital staff and study staff who contributed to the blood sample and data collection for this study.
Author information
Authors and Affiliations
Contributions
R.S.H. and M.G. designed the study and obtained financial support. R.S.H. drafted the manuscript with substantial contributions from M.G. E.P. performed overall project management, development, database development and oversaw laboratory analyses; F.J.H. performed statistical analyses; F.J.H. and E.P. performed bioinformatics analyses; J.V., B.O. and A.P. performed sample preparation; E.S. and S.E.K. performed curation and sample preparation of MRC ALL 97 trial samples; T.L. and E.R. managed and maintained UKCCS sample data; M.T. performed curation and sample preparation of UKCCS samples; J.M.A. and J.A.E.I. performed ascertainment, curation and sample preparation of the Northern Institute for Cancer Research case series. I.P.T. generated and managed UK CRC control genotypes. All authors contributed to the final paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 and Supplementary Figures 1–5 (PDF 334 kb)
Rights and permissions
About this article
Cite this article
Papaemmanuil, E., Hosking, F., Vijayakrishnan, J. et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet 41, 1006–1010 (2009). https://doi.org/10.1038/ng.430
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.430
This article is cited by
-
Genome-wide trans-ethnic meta-analysis identifies novel susceptibility loci for childhood acute lymphoblastic leukemia
Leukemia (2022)
-
Relationship between IKZF1 polymorphisms and the risk of acute lymphoblastic leukemia: a meta-analysis*
Oncology and Translational Medicine (2022)
-
Lymphoblastic Lymphoma: a Concise Review
Current Oncology Reports (2022)
-
The CEBPE rs2239633 genetic polymorphism on susceptibility to childhood acute lymphoblastic leukemia: an updated meta-analysis
Environmental Health and Preventive Medicine (2021)
-
Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia
Nature Reviews Immunology (2021)