Abstract
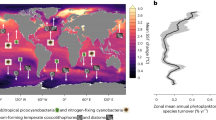
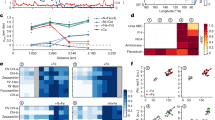
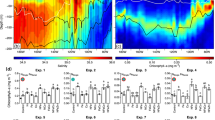
The availability of the micronutrient iron governs phytoplankton growth across much of the ocean, but the global iron cycle is changing rapidly due to accelerating acidification, stratification, warming and deoxygenation. These mechanisms of global change will cumulatively affect the aqueous chemistry, sources and sinks, recycling, particle dynamics and bioavailability of iron. Biological iron demand will vary as acclimation to environmental change modifies cellular requirements for photosynthesis and nitrogen acquisition and as adaptive evolution or community shifts occur. Warming, acidification and nutrient co-limitation interactions with iron biogeochemistry will all strongly influence phytoplankton dynamics. Predicting the shape of the future iron cycle will require understanding the responses of each component of the unique biogeochemistry of this trace element to many concurrent and interacting environmental changes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Moore, J. K., Lindsay, K., Doney, S. C., Long, M. C. & Misumi, K. Marine ecosystem dynamics and biogeochemical cycling in the Community Earth System Model CESM1(BGC). J. Clim. 26, 9291–9321 (2013).
Tagliabue, A. et al. How well do global ocean biogeochemistry models simulate dissolved iron distributions? Glob. Biogeochem. Cycles 30, 149–174 (2016). Compares 13 global ocean iron biogeochemistry models to iron measurement data sets from cross-basin transects, and finds that most do not match well, with suggestions for improvements.
Lis, H., Shaked, Y., Kranzler, C., Keren, N. & Morel, F. M. M. Iron bioavailability to phytoplankton: an empirical approach. ISME J. 9, 1003–1013 (2015).
Twining, B. S. & Baines, S. B. The trace metal composition of marine phytoplankton. Annu. Rev. Mar. Sci. 5, 191–215 (2013).
Boyd, P. W. & Ellwood, M. J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 3, 675–682 (2010).
Resing, J. A. et al. Basin scale transport of hydrothermal dissolved metals across the South Pacific Ocean. Nature 523, 200–203 (2015).
Armbrust, E. V. The life of diatoms in the world's oceans. Nature 459, 185–192 (2009).
Hutchins, D. A., Mulholland, M. R. & Fu, F.-X. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22, 128–145 (2009).
Mackie, D. S. et al. Biogeochemistry of iron in Australian dust: from eolian uplift to marine uptake. Geochem. Geophys. Geosyst. 9, Q03Q08 (2008).
Boyd, P. W., Arrigo, K. R., Strzepek, R. & van Dijken, G. L. Mapping iron demand provides insights into Southern Ocean supply mechanisms. J. Geophys. Res. 117, C06009 (2012).
Dutkiewicz, S., Scott, J. R. & Follows, M. J. Winners and losers: ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycles 27, 463–477 (2013).
Martinez-Garcia, A. et al. Iron fertilization of the Subantarctic Ocean during the last ice age. Science 43, 1347–1350 (2014).
Russell, J. L., Dixon, K. W., Gnanadesikan, A., Stouffer, R. J. & Toggweiler, J. R. The Southern Hemisphere westerlies in a warming world: propping open the door to the deep ocean. J. Clim. 19, 6382–6390 (2006).
Rignot, E., Jacobs, S., Mouginot, J. & Scheuchl, B. Ice shelf melting around Antarctica. Science 341, 266–270 (2013).
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Smith, K. L. Jr Free-drifting icebergs in the Southern Ocean: an overview. Deep-Sea Res. II 58, 1277–1284 (2011).
Dutkiewicz, S. et al. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Change 5, 1002–1006 (2015).
Boyd, P. W., Lennartz, S. T., Glover, D. M. & Doney, S. C. Biological ramifications of climate-change mediated oceanic multi-stressors. Nat. Clim. Change 5, 71–79 (2015).
Fishwick, M. P. et al. The impact of changing surface ocean conditions on the dissolution of aerosol iron. Glob. Biogeochem. Cycles 28, 1235–1250 (2014).
Sholkovitz, E. R., Sedwick, P. N., Church, T. M., Baker, A. R. & Powell, C. F. Fractional solubility of aerosol iron: synthesis of a global-scale data set. Geochim. Cosmochim. Acta 89, 173–189 (2012).
Ito, A. & Xu, L. Response of acid mobilization of iron-containing mineral dust to improvement of air quality projected in the future. Atmos. Chem. Phys. 14, 3441–3459 (2014).
Ito, T., Nenes, A., Johnson, M. S., Meskhidze, N. & Deutsch, C. Acceleration of oxygen decline in the tropical Pacific over the past decades by aerosol pollutants. Nat. Geosci. 9, 409–470 (2016).
Clegg, S. L. & Whitfield, M. A generalized model for the scavenging of trace metals in the open ocean—II: thorium scavenging. Deep-Sea Res. I 38, 91–120 (1991).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Wilhelm, S. W. et al. Elemental quotas and physiology of a southwestern Pacific Ocean plankton community as a function of iron availability. Aquat. Microb. Ecol. 68, 185–194 (2013).
Hutchins, D. A. et al. Phytoplankton iron limitation in the Humboldt Current and Peru Upwelling. Limnol. Oceanogr. 47, 997–1011 (2002).
Fu, F.-X. et al. Interactions between changing pCO2, N2 fixation, and Fe limitation in the marine unicellular cyanobacterium Crocosphaera. Limnol. Oceanogr. 53, 2472–2484 (2008).
King, A. L., Sañudo-Wilhelmy, S. A., Leblanc, K., Hutchins, D. A. & Fu, F.-X. CO2 and vitamin B12 interactions determine bioactive trace metal requirements of a subarctic Pacific diatom. ISME J. 5, 1388–1396 (2011).
Xu, K., Fu, F.-X. & Hutchins, D. A. Comparative responses of two dominant Antarctic phytoplankton taxa to interactions between ocean acidification, warming, irradiance, and iron availability. Limnol. Oceanogr. 59, 919–931 (2014).
Zhu, Z. et al. A comparative study of iron and temperature interactive effects on diatoms and Phaeocystis antarctica from the Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 550, 39–51 (2016).
Boyd, P. W. et al. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617 (2007).
Quigg, A. et al. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425, 291–294 (2003).
Quigg, A., Irwin, A. J. & Finkel, Z. V. Evolutionary inheritance of elemental stoichiometry in phytoplankton. Proc. R. Soc. B 278, 526–534 (2011).
Orr, J. C. et al. Anthropogenic acidification over the twenty first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Shadwick, E. H., Trull, T. W., Thomas, H. & Gibson, J. A. E. Vulnerability of polar oceans to ocean acidification: comparison of Arctic and Antarctic seasonal cycles. Sci. Rep. 3, 2339 (2013).
Tortell, P. D. et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 35, L04605 (2008).
Feng, Y. et al. Interactive effects of iron, irradiance and CO2 on Ross Sea phytoplankton. Deep-Sea Res. I 57, 368–383 (2010).
Hopkinson, B. M. et al. The effect of CO2 on the photosynthetic physiology of phytoplankton in the Gulf of Alaska. Limnol. Oceanogr. 55, 2011–2024 (2010).
Hoppe, C. J. M. et al. Iron limitation modulates ocean acidification effects on Southern Ocean phytoplankton communities. PLoS ONE 8, e79890 (2013).
Sugie, K. et al. Synergistic effects of pCO2 and iron availability on nutrient consumption ratio of the Bering Sea phytoplankton community. Biogeosciences 10, 6309–6321 (2013).
Yoshimura, T. et al. Organic matter production response to CO2 increase in open subarctic plankton communities: comparison of six microcosm experiments under iron-limited and -enriched bloom conditions. Deep-Sea Res. I 94, 1–14 (2014).
Sohm, J. A., Webb, E. A. & Capone, D. G. Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 1–10 (2011).
Raven, J. A. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 109, 279–287 (1988).
Kustka, A. B. et al. Iron requirements for dinitrogen- and ammonium supported growth in cultures of Trichodesmium (IMS 101): comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnol. Oceanogr. 48, 1869–1884 (2003).
Hutchins, D. A., Fu, F.-X., Webb, E. A., Walworth, N. & Tagliabue, A. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nat. Geosci. 6, 790–795 (2013).
Fu, F.-X. et al. Differing responses of marine N2-fixers to warming and consequences for future diazotroph community structure. Aquat. Microb. Ecol. 72, 33–46 (2014).
Hutchins, D. A. et al. Irreversibly increased N2 fixation in Trichodesmium experimentally adapted to high CO2 . Nat. Commun. 6, 8155 (2015).
McMahon, K. W., McCarthy, M. D., Sherwood, O. A., Larsen, T. & Guilderson, T. P. Millennial-scale plankton regime shifts in the subtropical North Pacific Ocean. Science 350, 1530–1533 (2015).
Shi, D., Kranz, S. A., Kim, J.-M. & Morel, F. M. M. Ocean acidification slows nitrogen fixation and growth in dominant diazotroph Trichodesmium under low-iron conditions. Proc. Natl Acad. Sci. USA 109, E3094–E3100 (2012).
Walworth, N. G. et al. Mechanisms of increased Trichodesmium fitness under iron and phosphorus co-limitation in the present and future ocean. Nat. Commun. 7, 12081 (2016). Examines the unique physiologies and proteomes of iron and phosphorus co-limited Trichodesmium and their interactions with adaptation to high CO 2.
Rose, J. M. et al. Synergistic effects of iron and temperature on Antarctic phytoplankton and microzooplankton assemblages. Biogeosciences 6, 3131–3147 (2009). Demonstrates the markedly nonlinear responses of a Ross Sea diatom community to the interactive effects of iron and warming.
Boyd, P. W. et al. Physiological responses of a Southern Ocean diatom to complex future ocean conditions. Nat. Clim. Change 6, 207–216 (2016). Investigates how an Antarctic diatom responds to multivariate changes in five climate change factors, and finds that warming and iron are the most influential.
Monteiro, F. M., Dutkiewicz, S. & Follows, M. J. Biogeographical controls on the marine nitrogen fixers. Glob. Biogeochem. Cycles 25, GB2003 (2011).
Maranon, E. et al. Resource supply overrides temperature as a controlling factor of marine phytoplankton growth. PLoS ONE 9, e99312 (2004).
Sunda, W. G. & Huntsman, S. A. Interactive effects of light and temperature on iron limitation in a marine diatom: implications for marine productivity and carbon cycling. Limnol. Oceanogr. 56, 1475–1488 (2011). Shows that diatom iron use efficiency increases as a function of temperature and light.
Clarke, A. Life in cold water: the physiological ecology of polar marine ectotherms. Oceanogr. Mar Biol. Annu. Rev. 21, 341–453 (1983).
Toseland, A. et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Change 3, 979–984 (2013). Finds that phytoplankton protein synthesis per ribosome increases with warming, with implications for nutrient requirements and ratios.
Saito M. A. et al. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc. Natl Acad. Sci. USA 108, 2184–2189 (2011).
Laufkötter, C. et al. Drivers and uncertainties of future global marine primary production in marine ecosystem models. Biogeosciences 12, 6955–6984 (2015).
Saito, M. A., Goepfert, T. J. & Ritt, J. T. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnol. Oceanogr. 53, 276–290 (2008).
Pelletier, J. D. et al. Forecasting the response of Earth's surface to future climatic and land use changes: a review of methods and research needs. Earth's Future 3, 220–251 (2015).
Beman, J. M. et al. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc. Natl Acad. Sci. USA 108, 208–213 (2011).
Muggli, D. L., Lecourt, M. & Harrison, P. J. Effects of iron and nitrogen source on the sinking rate, physiology and metal composition of an oceanic diatom from the subarctic Pacific. Mar. Ecol. Prog. Ser. 132, 215–227 (1996).
Muggli, D. L. & Harrison, P. J. Effects of nitrogen source on the physiology and metal nutrition of Emiliania huxleyi grown under different iron and light conditions. Mar. Ecol. Prog. Ser. 130, 255–267 (1996).
Hutchins, D. A. & Bruland, K. W. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393, 561–564 (1998).
Hutchins, D. A. et al. Control of phytoplankton growth by iron and silicic acid availability in the subantarctic Southern Ocean: experimental results from the SAZ project. J. Geophys. Res. Oceans 106, 559–572 (2002).
Mills, M. M., Ridame, C., Davey, M., LaRoche, J. & Gelder, R. J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294 (2004).
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013).
Snow, J. T. et al. Environmental controls on the biogeography of diazotrophy and Trichodesmium in the Atlantic Ocean. Glob. Biogeochem. Cycles 29, 865–884 (2015).
Garcia, N. S., Fu, F.-X., Sedwick, P. N. & Hutchins, D. A. Iron deficiency increases growth and nitrogen fixation rates of phosphorus-deficient marine cyanobacteria. ISME J. 9, 238–245 (2015).
Hutchins, D. A. & Fu, F.-X. in Nitrogen in the Marine Environment 2nd edn (eds Capone, D. G. et al.) 1627–1653 (Elsevier, 2008).
Ward, B. A., Dutkiewicz, S., Moore, C. M. & Follows, M. J. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr. 58, 2059–2075 (2013).
Letscher, R. T. & Moore, J. K. Preferential remineralization of dissolved organic phosphorus and non-Redfield DOM dynamics in the global ocean: impacts on marine productivity, nitrogen fixation, and carbon export. Glob. Biogeochem. Cycles 29, 325–340 (2015).
Strzepek, R. F. & Harrison, P. J. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431, 689–692 (2004). Demonstrates that diatoms from iron-limited oceanic regimes have evolved a photosynthetic apparatus that requires much less iron compared with coastal species.
Strzepek, R., Maldonado, M., Hunter, K., Frew, R. & Boyd, P. W. Adaptive strategies by Southern Ocean phytoplankton to lessen iron limitation: uptake of organically complexed iron and reduced cellular iron requirements. Limnol. Oceanogr. 56, 1983–2002 (2012).
Sunda, W. G. & Huntsman, S. A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 50, 189–206 (1995).
Marchetti, A., Maldonado, M. T., Lane, E. S. & Harrison, P. J. Iron requirements of the pennate diatom Pseudo-nitzschia: comparison of oceanic (HNLC) and coastal species. Limnol. Oceanogr. 51, 2092–2101 (2006).
King A. L. et al. A comparison of biogenic iron quotas during a diatom spring bloom using multiple approaches. Biogeosciences 9, 667–687 (2012).
Lohbeck, K. T., Riebesell, U. & Reusch, T. B. H. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351 (2012).
Schaum, C. E. & Collins, S. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B 281, 20141486 (2014).
Tagliabue, A. et al. Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing. Nat. Geosci. 7, 314–320 (2014).
Meehl, G. A., Arblaster, J. M., Bitz, C. M., Chung, C. T. Y. & Teng, H. Antarctic sea-ice expansion between 2000 and 2014 driven by tropical Pacific decadal climate variability. Nat. Geosci. 9, 590–595 (2016).
Bruland, K. W., Donat J. R. & Hutchins, D. A. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol. Oceanogr. 36, 1555–1577 (1991).
Hutchins, D. A., Witter, A. E., Butler, A. & Luther, G. W. III Competition among marine phytoplankton for different chelated iron species. Nature 400, 858–861 (1999).
Gledhill, M. & Buck, K. N. The organic complexation of iron in the marine environment: a review. Front. Microbiol. 3, 69 (2012).
Breitbarth, E. et al. Ocean acidification affects iron speciation during a coastal seawater mesocosm experiment. Biogeosciences 7, 1065–1073 (2010).
Capone D. & Hutchins D. A. Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nat. Geosci. 6, 711–717 (2013).
Millero, F. J., Sotolongo, S. & Izaguirre, M. The oxidation kinetics of Fe(II) in seawater. Geochim. Cosmochim. Acta 51, 793–801 (1987).
Croot, P. L. et al. Retention of dissolved iron and Fe II in an iron induced Southern Ocean phytoplankton bloom. Geophys. Res. Lett. 28, 3425–3428 (2001).
Kustka, A. B. et al. The influence of iron and siderophores on eukaryotic phytoplankton growth rates and community composition in the Ross Sea. Mar. Chem. 173, 195–207 (2015).
Barbeau, K., Rue, E. L., Bruland, K. W. & Butler, A. Photochemical cycling of iron in the surface ocean mediated by microbial iron (III)-binding ligands. Nature 413, 409–413 (2001). Photoreduction of iron–ligand complexes plays a critical role in making organically complexed iron available to the biota.
Hoffmann, L. J., Breitbarth, E., Boyd, P. W. & Hunter, K. A. Influence of ocean warming and acidification on trace metal biogeochemistry. Mar. Ecol. Prog. Ser. 470, 191–205 (2012).
Joint, I., Doney, S. C. & Karl, D. M. Will ocean acidification affect marine microbes? ISME J. 5, 1–7 (2011).
Zark, M., Riebesell, U. & Dittmar, T. Effects of ocean acidification on marine dissolved organic matter are not detectable over the succession of phytoplankton blooms. Sci. Adv. 1, e1500531 (2015).
Shi, D., Xu, Y., Hopkinson, B. M. & Morel, F. M. M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679 (2010).
Gledhill, M., Achterberg, E. P., Li, K., Mohamed, K. N. & Rijkenberg, M. J. A. Influence of ocean acidification on the complexation of iron and copper by organic ligands in estuarine waters. Mar. Chem. 177, 421–433 (2015).
Stockdale, A., Tipping, E., Lofts, S. & Mortimer, R. J. G. Effect of ocean acidification on organic and inorganic speciation of trace metals. Environ. Sci. Technol. 50, 1906–1913 (2016).
Acknowledgements
The authors thank F. Fu and S. Sanudo-Wilhelmy for help with obtaining unpublished Fe quota data, and J. Brown and the University of Southern California Wrigley Institute of Environmental Sciences for their generous assistance with graphics. Support was provided by US National Science Foundation grants OCE 1260490 and OCE 1538525 to D.A.H., Australian Research Council Australian Laureate Fellowship project FL160100131 and Antarctic Climate and Ecosystems Cooperative Research Centre funding to P.W.B. and the Australian Research Council's Special Research Initiative for Antarctic Gateway Partnership Project ID SR140300001 to both authors.
Author information
Authors and Affiliations
Contributions
D.A.H. and P.W.B. contributed equally to conceiving and developing the material presented, and to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Marine phytoplankton and the changing ocean iron cycle (PDF 340 kb)
Rights and permissions
About this article
Cite this article
Hutchins, D., Boyd, P. Marine phytoplankton and the changing ocean iron cycle. Nature Clim Change 6, 1072–1079 (2016). https://doi.org/10.1038/nclimate3147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate3147
This article is cited by
-
The role of biota in the Southern Ocean carbon cycle
Nature Reviews Earth & Environment (2024)
-
Jellyfish detritus supports niche partitioning and metabolic interactions among pelagic marine bacteria
Microbiome (2023)
-
Short-term acidification promotes diverse iron acquisition and conservation mechanisms in upwelling-associated phytoplankton
Nature Communications (2023)
-
The underappreciated role of anthropogenic sources in atmospheric soluble iron flux to the Southern Ocean
npj Climate and Atmospheric Science (2022)
-
Enhanced silica export in a future ocean triggers global diatom decline
Nature (2022)