Abstract
Chimaeroid fishes (Holocephali) are one of the four principal divisions of modern gnathostomes (jawed vertebrates). Despite only 47 described living species1, chimaeroids are the focus of resurgent interest as potential archives of genomic data2 and for the unique perspective they provide on chondrichthyan and gnathostome ancestral conditions. Chimaeroids are also noteworthy for their highly derived body plan1,3,4. However, like other living groups with distinctive anatomies5, fossils have been of limited use in unravelling their evolutionary origin, as the earliest recognized examples already exhibit many of the specializations present in modern forms6,7. Here we report the results of a computed tomography analysis of Dwykaselachus, an enigmatic chondrichthyan braincase from the ~280 million year old Karoo sediments of South Africa8. Externally, the braincase is that of a symmoriid shark9,10,11,12,13 and is by far the most complete uncrushed example yet discovered. Internally, the morphology exhibits otherwise characteristically chimaeroid specializations, including the otic labyrinth arrangement and the brain space configuration relative to exceptionally large orbits. These results have important implications for our view of modern chondrichthyan origins, add robust structure to the phylogeny of early crown group gnathostomes, reveal preconditions that suggest an initial morpho-functional basis for the derived chimaeroid cranium, and shed new light on the chondrichthyan response to the extinction at the end of the Devonian period.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Didier, D. A., Kemper, J. M. & Ebert, D. A. in Biology of Sharks and Their Relatives (eds Carrier, J. C., Musick, J. A. & Heithaus, M. R. ) 97–122 (CRC, 2012)
Venkatesh, B. et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179 (2014)
Patterson, C. The phylogeny of the chimaeroids. Phil. Trans. R. Soc. Lond. B 249, 101–219 (1965)
Stahl, B. J. in Handbook of Paleoichthyology Vol. 4 (ed. Schultze, H.-P. ) (Friedrich Pfeil, 1999)
Simmons, N. B., Seymour, K. L., Habersetzer, J. & Gunnell, G. F. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451, 818–821 (2008)
Grogan, E. D., Lund, R. & Greenfest-Allen, E. in Biology of Sharks and Their Relatives (eds Carrier, J. C., Musick, J. A. & Heithaus, M. R. ) 3–29 (CRC, 2012)
Finarelli, J. A. & Coates, M. I. Chondrenchelys problematica (Traquair, 1888) redescribed: a Lower Carboniferous, eel-like holocephalan from Scotland. Earth Env. Sci. Trans. R. Soc. Edinb. 105, 1–25 (2014)
Oelofsen, B. W. in Indo-Pacific Fish Biology: Proceedings of the SecondInternational Conference on Indo-Pacific Fishes (eds Uyeno, T., Arai, R., Taniuchi, T. & Matsuura, K. ) 107–124 (Ichthyological Society of Japan, 1986)
Maisey, J. G. The braincase in Paleozoic symmoriiform and cladoselachian sharks. Bull. Am. Mus. Nat. Hist. 307, 1–122 (2007)
Coates, M. I. & Sequeira, S. E. K. The braincase of a primitive shark. Trans. R. Soc. Edinb. Earth Sci. 89, 63–85 (1998)
Zangerl, R. & Case, G. R. Cobelodus aculeatus (Cope), an anacanthous shark from the Pennsylvanian black shales of North America. Palaeontographica A 154, 107–157 (1976)
Williams, M. E. The “cladodont level” sharks of the Pennsylvanian black shales of central North America. Palaeontographica A 190, 83–158 (1985)
Pradel, A., Maisey, J. G., Tafforeau, P., Mapes, R. H. & Mallatt, J. A Palaeozoic shark with osteichthyan-like branchial arches. Nature 509, 608–611 (2014)
Bangert, B., Stollhofen, H., Lorenz, V. & Armstrong, R. The geochronology and significance of ash-fall tuffs in the glaciogenic Carboniferous-Permian Dwyka Group of Namibia and South Africa. J. Afr. Earth Sci. 29, 33–49 (1999)
McKay, M. P. et al. U-PB zircon tuff geochronology from the Karoo Basin, South Africa: implications of zircon recycling on stratigraphic age controls. Int. Geol. Rev. 57, 393–410 (2015)
De Beer, G. R. & Moy-Thomas, J. A. On the skull of Holocephali. Phil. Trans. R. Soc. Lond. B 224, 287–312 (1935)
Schaeffer, B. The xenacanth shark neurocranium, with comments on elasmobranch monophyly. Bull. Am. Mus. Nat. Hist. 169, 1–66 (1981)
Pradel, A. Skull and brain anatomy of Late Carboniferous Sibyrhynchidae (Chondrichthyes, Iniopterygia) from Kansas and Oklahoma (USA). Geodiversitas 32, 595–661 (2010)
Maisey, J. G., Miller, R. & Turner, S. The braincase of the chondrichthyan Doliodus from the Lower Devonian Campbellton Formation of New Brunswick, Canada. Acta Zoologica 90 (Suppl. 1), 109–122 (2009)
Pradel, A., Didier, D., Casane, D., Tafforeau, P. & Maisey, J. G. Holocephalan embryo provides new information on the evolution of the glossopharyngeal nerve, metotic fissue and parachordal plate in gnathostomes. PLoS One 8, e66988 (2013)
Giles, S. & Friedman, M. Virtual reconstruction of endocast anatomy in early ray-finned fishes (Osteichthyes, Actinopterygii). J. Paleontol. 88, 636–651 (2014)
Howard, L. E. et al. Functional nasal morphology of chimaerid fishes. J. Morphol. 274, 987–1009 (2013)
Coates, M. I. & Sequeira, S. E. K. in Major Events in Early Vertebrate Evolution (ed. Ahlberg, P. E. ) 241–262 (Taylor & Francis, 2001)
Janvier, P. Early Vertebrates (Clarendon, 1996)
Giles, S., Friedman, M. & Brazeau, M. D. Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome. Nature 520, 82–85 (2015)
Friedman, M. & Sallan, L. C. Five hundred million years of extinction and recovery: a Phanerozoic survey of large-scale diversity patterns in fishes. Palaeontology 55, 707–742 (2012)
Pradel, A., Tafforeau, P., Maisey, J. G. & Janvier, P. A new paleozoic Symmoriiformes (Chondrichthyes) from the late Carboniferous of Kansas (USA) and cladistic analysis of early chondrichthyans. PLoS One 6, e24938 (2011)
Cronin, T. W., Johnsen, S., Marshall, N. J. & Warrant, E. J. Visual Ecology (Princeton Univ. Press, 2014)
Schmitz, L. & Wainwright, P. C. Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evol. Biol. 11, 338 (2011)
Lisney, T. J. A review of the sensory biology of chimaeroid fishes (Chondrichthyes; Holocephali). Rev. Fish Biol. Fish. 20, 571–990 (2010)
Swofford, D. L. PAUP*: Phylogenetic Analysis Using Parsimony (*And Other Methods) v.4.0a147 (Sinauer Associates, 2003)
Felsenstein, J. Confidence-limits on phylogenies—an approach using the bootstrap. Evolution 39, 783–791 (1985)
Bremer, K. The limits of amino-acid sequence data in angiosperm phylogenetic reconstruction. Evolution 42, 795–803 (1988)
Eriksson, T. AutoDecay Version 5.0. (2001)
Rannala, B. & Yang, Z. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311 (1996)
Yang, Z. & Rannala, B. Bayesian phylogenetic inference using DNA sequences: a Markov Chain Monte Carlo Method. Mol. Biol. Evol. 14, 717–724 (1997)
Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001)
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003)
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012)
Gradstein, F. M., Ogg, J. G., Schmitz, M. & Ogg, G. The Geologic Time Scale 2012 (Elsevier, 2012)
Brazeau, M. D. & Friedman, M. The origin and early phylogenetic history of jawed vertebrates. Nature 520, 490–497 (2015)
Young, G. C. Devonian sharks from south-eastern Australia and Antarctica. Palaeontology 25, 817–843 (1982)
Gaudin, T. J. A re-examination of elasmobranch monophyly and chondrichthyan phylogeny. Jb. Geol. Palaont. Abh. 182, 133–160 (1991)
Lund, R. & Grogan, E. D. Relationships of the Chimaeriformes and the basal radiation of the Chondrichthyes. Rev. Fish Biol. Fish. 7, 65–123 (1997)
Ginter, M., Hampe, O. & Duffin, C. in Handbook of Paleoichthyology Vol. 3D (ed. Schultze, H.-P. ) (Friedrich Pfeil, 2010)
Davis, S. P., Finarelli, J. A. & Coates, M. I. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature 486, 247–250 (2012)
Zhu, M. et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502, 188–193 (2013)
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P. & Ahlberg, P. E. A. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507, 500–503 (2014)
Janvier, P. & Pradel, A. in Physiology of Elasmobranch Fishes: Structure and Interaction with Environment (eds Shadwick, R. E., Farrell, A. P. & Brauner, C. J. ) (Academic, 2016)
Maisey, J. G. Chondrichthyan phylogeny: a look at the evidence. J. Vertebr. Paleontol. 4, 359–371 (1984)
Zangerl, R. in Handbook of Paleoichthyology Vol. 3A (ed. Schultze, H.-P. ) (Gustav Fischer, 1981)
Zangerl, R. in Mazon Creek Fossils (ed. Nitecki, M. H. ) 449–500 (Academic, 1979)
Tapanila, L. et al. Jaws for a spiral-tooth whorl: CT images reveal novel adaptation and phylogeny in fossil Helicoprion. Biol. Lett. 9, 20130057 (2013)
Benton, M. J. et al. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica 18, 1–116 (2015)
Darras, L., Derycke, C., Blieck, A. & Vachard, D. The oldest holocephalan (Chondrichthyes) from the Middle Devonian of the Boulonnais (Pas-de-Calais, France). C. R. Palevol 7, 297–304 (2008)
Gardiner, B. G. The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bull. Br. Mus. Nat. Hist. 37, 173–428 (1984)
Acknowledgements
We thank the Evolutionary Studies Institute micro-CT scanning unit at the University of the Witwatersrand in Johannesburg, South Africa, for provision of scanning facilities and K. Carlson and K. Jakata for technical assistance. We thank R. Smith and the staff of the South African Museum, Cape Town, for access and loan of specimen. We thank A. Gillis for the donation of Callorhinchus milii embryos and hatchlings. This work was supported by grants DEB-0917922 and DEB-1541491 from the National Science Foundation (to M.I.C.), and a research grant from the National Research Foundation/Department of Science and Technology South African Centre of Excellence in Palaeosciences (to R.W.G.).
Author information
Authors and Affiliations
Contributions
This project was conceived by M.I.C. and R.W.G. and developed by M.I.C., R.W.G., J.A.F. and K.E.C. CT scanning of Moythomasia durgaringa was conducted by K.T.; renderings and animations of CT scans and graphics were completed by K.T. with input from M.I.C., K.E.C. and J.A.F. C. milii preparation and staining were completed by K.E.C. Phylogenetic data were collected by M.I.C., K.E.C. and J.A.F. Phylogenetic analyses were completed by J.A.F. and M.I.C. Morphometric data were collected by M.I.C. and K.E.C.; analysis was completed by M.I.C., K.E.C. and J.A.F. Manuscript and supplementary text preparation was undertaken by M.I.C., R.W.G. and J.A.F. with input from K.E.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Friedman, P. Janvier and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
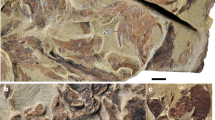
Extended Data Figure 1 D. oosthuizeni Oelofsen 1986 SAM K584, the nodule.
a, Ventral part, showing infilled orbits, ethmoid region floor and floor of otic and occipital regions in dorsal view; anterior to left. b, Nodule in lateral view, showing division between dorsal and ventral sections. c, Dorsal part, showing infilled nasal capsules, supraorbital shelves, and endocranium in ventral view. Anterior to right in b and c. d, Area of tesselate calcified cartilage.
Extended Data Figure 2 Comparison of symmoriiform braincases.
a, FMNH PF 13242, lateral view, drawn from figure 6 in ref. 9. b, FMNH 13242, ventral view, drawn from figure 8 in ref. 9. c, Cobelodus, FMNH PF 7345, drawn from figure 5b in ref. 11. d, Ozarcus, AMNH FF 20544, lateral view, drawn from extended data figure 2h in ref. 13, grey area signifies material missing in a. e, Ozarcus, AMNH FF 20544, ventral view, adapted from extended data figure 2h in ref. 13, Nature Publishing Group. f, Dwykaselachus, lateral view restored. g, Dwykaselachus, ventral view restored. h, Akmonistion, NMS 1981.63.22C, ventral view, drawn from figure 1c in ref. 10.
Extended Data Figure 3 Ethmoid region of Dwykaselachus based on CT rendering of SAM K584.
a, Anterior view with material anterior to blue line in b removed. b, Dorsal view of nasal capsules and anterior portion of interorbital space, modelled as if sectioned horizontally at mid-capsular level; horizontal blue line indicates level of sections shown in a and c. c, Anterior view of endocranial spaces incompletely infilled to show pattern of interconnecting chambers (model sectioned vertically at mid-nasal capsule level, as indicated by blue line in b). The subnasal space may have formed a cartilage-filled antorbital process (see also Extended Data Fig. 5a), but the communication with the nasal capsule via the subnasal fenestra raises the possibility that it housed an accessory nasal duct, resembling those in modern chimaeroids1,22; arrows suggest possible direction of water flow. d, Anterolateral and ventral aspect. e, Posterior view of ethmoid region, model sectioned across anterior of orbit. f, g, Line drawings of d and e, respectively.
Extended Data Figure 4 Endocast morphology of D. oosthuizeni SAM K5840.
a, b, CT rendering of cranial endocast, semicircular canals and ampullae, and path of superficial ophthalmic nerves in dorsal view (a) and in lateral view (b).
Extended Data Figure 5 D. oosthuizeni SAM K5840 braincase.
a, CT rendering in ventrolateral view. b, Rendering of braincase roof, posterior to level of postorbital process, with occipital arch removed to show posterior tectum.
Extended Data Figure 6 D. oosthuizeni SAM K5840 braincase.
a, Anterior view of CT rendering of postorbital wall, as if the braincase is cross-sectioned (clipped) at mid-orbit level. b, Anterolateral view of same. Removal of ethmoid region permits an unobstructed view. c, d, CT rendering of braincase in posterior view (c) and posterolateral view (d).
Extended Data Figure 7 D. oosthuizeni SAM K5840 braincase.
Medial view of sagittal section of CT rendered neurocranium, showing endocranial spaces.
Extended Data Figure 8 Symmoriiform and osteichthyan (actinopterygian) occipital arches.
a, b, Dwykaselachus in lateral and posterior views; model based on mirrored right side CT of SAM K5840. c, d, Moythomasia durgaringa Gardiner & Bartram in lateral and posterior views, model based on CT of MV P222915 (dorsal portion of occipital plate missing). Blue line in a and c indicates profile of braincase posterior extremity of the otic capsule; profile for Moythomasia, drawn from ref. 56. In both examples, the dorsal part of the occipital arch does not project anteriorly between the otic capsules (unlike elasmobranch conditions).
Extended Data Figure 9 Dwykaselachus and Callorhinchus crania rendered semi-transparent in anterolateral view.
a, b, Dwykaselachus, model based on CT of SAM K5840 (a) and Callorhinchus, revealing relation of superficial ophthalmic trunks to forebrain, and forebrain level relative to dorsally prominent mesencephalon (b). c, Callorhinchus CT coronal preorbital slice linked to framed section in b; ellipses (black line) indicate orbit size.
Extended Data Figure 10 Consensus trees from phylogenetic analyses.
a, Maximum parsimony analysis. Strict consensus of 240 most parsimonious trees; node numbers ringed (character state transitions listed in Supplementary Table 2); numbers above branches are Bremer support scores and numbers below are bootstrap values. Character state summaries (grey boxes) list neurocranial changes at basal nodes within the total group Holocephali. b, Bayesian analysis. Majority rule consensus tree; numbers above branches are the posterior probabilities, measured as proportion of sampled trees in which that topology was recovered. Nodes ringed in red are recovered in both the maximum parsimony and Bayesian analyses.
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Morphometric and Phylogenetic Analysis and Supplementary references. (PDF 1445 kb)
Supplementary Data
This zipped file contains the data matrix nexus file. (ZIP 6 kb)
Dwykaselachus braincase rotate and reveal endocast
Dwykaselachus braincase rotate and reveal endocast. (MP4 13894 kb)
Callorhinchus subadult skull rotate and internal morphology reveal
Callorhinchus subadult skull rotate and internal morphology reveal, illustrating palatal fusion to braincase, large orbits positioned mostly dorsal to forebrain, superficial ophthalmic nerve paths and associated ethmoid canal, brain and otic labyrinth configuration. (MP4 17089 kb)
Rights and permissions
About this article
Cite this article
Coates, M., Gess, R., Finarelli, J. et al. A symmoriiform chondrichthyan braincase and the origin of chimaeroid fishes. Nature 541, 208–211 (2017). https://doi.org/10.1038/nature20806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20806