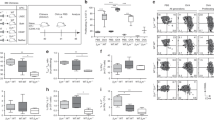
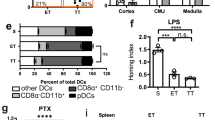
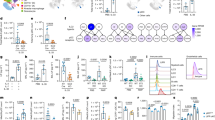
Abstract
While patrolling the body in search of foreign antigens, naive lymphocytes continuously circulate from the blood, through the lymph nodes, into the lymphatic vessels and back to the blood1,2. This process, called lymphocyte recirculation, provides the body with effective immune surveillance for foreign invaders and for alterations to the body’s own cells. However, the mechanisms that regulate lymphocyte recirculation during homeostasis remain incompletely characterized. Here we show that dendritic cells (DCs), which are well known for their role in antigen presentation to T lymphocytes3, control the entry of naive lymphocytes to lymph nodes by modulating the phenotype of high endothelial venules (HEVs), which are blood vessels specialized in lymphocyte recruitment2,4,5. We found that in vivo depletion of CD11c+ DCs in adult mice over a 1-week period induces a reduction in the size and cellularity of the peripheral and mucosal lymph nodes. In the absence of DCs, the mature adult HEV phenotype reverts to an immature neonatal phenotype, and HEV-mediated lymphocyte recruitment to lymph nodes is inhibited. Co-culture experiments showed that the effect of DCs on HEV endothelial cells is direct and requires lymphotoxin-β-receptor-dependent signalling. DCs express lymphotoxin, and DC-derived lymphotoxin is important for lymphocyte homing to lymph nodes in vivo. Together, our results reveal a previously unsuspected role for DCs in the regulation of lymphocyte recirculation during immune surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996)
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003)
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998)
Girard, J. P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995)
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nature Rev. Immunol. 4, 360–370 (2004)
Rosen, S. D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004)
Streeter, P. R., Rouse, B. T. & Butcher, E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988)
Uchimura, K. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nature Immunol. 6, 1105–1113 (2005)
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nature Immunol. 6, 1096–1104 (2005)
Malý, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996)
Hendriks, H. R. & Eestermans, I. L. Disappearance and reappearance of high endothelial venules and immigrating lymphocytes in lymph nodes deprived of afferent lymphatic vessels: a possible regulatory role of macrophages in lymphocyte migration. Eur. J. Immunol. 13, 663–669 (1983)
Mebius, R. E., Streeter, P. R., Breve, J., Duijvestijn, A. M. & Kraal, G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 115, 85–95 (1991)
Swarte, V. V. et al. Regulation of fucosyltransferase-VII expression in peripheral lymph node high endothelial venules. Eur. J. Immunol. 28, 3040–3047 (1998)
Mebius, R. E. et al. Expression of GlyCAM-1, an endothelial ligand for L-selectin, is affected by afferent lymphatic flow. J. Immunol. 151, 6769–6776 (1993)
Lacorre, D. A. et al. Plasticity of endothelial cells: rapid dedifferentiation of freshly isolated high endothelial venule endothelial cells outside the lymphoid tissue microenvironment. Blood 103, 4164–4172 (2004)
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002)
Zaft, T., Sapoznikov, A., Krauthgamer, R., Littman, D. R. & Jung, S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J. Immunol. 175, 6428–6435 (2005)
Mebius, R. E., Streeter, P. R., Michie, S., Butcher, E. C. & Weissman, I. L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3− cells to colonize lymph nodes. Proc. Natl Acad. Sci. USA 93, 11019–11024 (1996)
Von Andrian, U. H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996)
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003)
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004)
Liao, S. & Ruddle, N. H. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 177, 3369–3379 (2006)
Browning, J. L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005)
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000)
Janzer, R. C. & Raff, M. C. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325, 253–257 (1987)
GeurtsvanKessel, C. H. et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 206, 2339–2349 (2009)
Muniz, L. R., Pacer, M. E., Lira, S. A. & Furtado, G. C. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J. Immunol. 187, 828–834 (2011)
Webster, B. et al. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 203, 1903–1913 (2006)
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999)
Cayrol, C. et al. The THAP-zinc finger protein THAP1 regulates endothelial cell proliferation through modulation of pRB/E2F cell-cycle target genes. Blood 109, 584–594 (2007)
Körner, H. et al. Distinct roles for lymphotoxin-α and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27, 2600–2609 (1997)
Kuprash, D. V. et al. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol. Cell. Biol. 22, 8626–8634 (2002)
Sapoznikov, A. et al. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nature Immunol. 9, 388–395 (2008)
Moussion, C., Ortega, N. & Girard, J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE 3, e3331 (2008)
Chen, Q. et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nature Immunol. 7, 1299–1308 (2006)
Carriere, V. et al. Cancer cells regulate lymphocyte recruitment and leukocyte–endothelium interactions in the tumor-draining lymph node. Cancer Res. 65, 11639–11648 (2005)
Flaishon, L. et al. Anti-inflammatory effects of an inflammatory chemokine: CCL2 inhibits lymphocyte homing by modulation of CCL21-triggered integrin-mediated adhesions. Blood 112, 5016–5025 (2008)
Acknowledgements
We are grateful to S. Rosen, J. Lowe and J. Browning for the gift of antibodies, to H. Korner, T. Winkler, E. Donnadieu, M. Lipp, O. Lantz, B. Ryffel, L. Brault, S. A. Nedospasov and S. Jung for providing mice, to J. van Meerwijk for critical reading of the manuscript, and to N. Ortega, E. Bellard and F. El-Fahqui-Olive for help with immunohistochemistry, intravital microscopy and cell sorting, respectively. We thank the IPBS TRI imaging facility, A. Dujardin and the IPBS animal and transgenic facilities for help with animal experiments, and F. Viala for preparation of the figures. This work was supported by grants from the Ligue Nationale Contre le Cancer (Equipe labellisée Ligue 2009 to J.-P.G.) and the Association pour la Recherche contre le Cancer (ARC, equipment 8505). C.M. was supported by fellowships from the French Ministry of Research, the ARC and Fondation RITC.
Author information
Authors and Affiliations
Contributions
C.M. designed the study, performed all of the experiments and analysed the data. J.-P.G. designed the study, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with legends, a Supplementary Discussion and additional references. (PDF 3064 kb)
Rights and permissions
About this article
Cite this article
Moussion, C., Girard, JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479, 542–546 (2011). https://doi.org/10.1038/nature10540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10540
This article is cited by
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Quantitation of lymphatic transport mechanism and barrier influences on lymph node-resident leukocyte access to lymph-borne macromolecules and drug delivery systems
Drug Delivery and Translational Research (2021)
-
Apatinib combined with PD-L1 blockade synergistically enhances antitumor immune responses and promotes HEV formation in gastric cancer
Journal of Cancer Research and Clinical Oncology (2021)
-
Emerging roles of infiltrating granulocytes and monocytes in homeostasis
Cellular and Molecular Life Sciences (2020)
-
Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism
Cellular & Molecular Immunology (2019)