Abstract
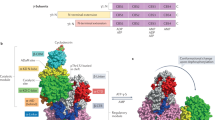
The heterotrimeric AMP-activated protein kinase (AMPK) has a key role in regulating cellular energy metabolism; in response to a fall in intracellular ATP levels it activates energy-producing pathways and inhibits energy-consuming processes1. AMPK has been implicated in a number of diseases related to energy metabolism including type 2 diabetes, obesity and, most recently, cancer2,3,4,5,6. AMPK is converted from an inactive form to a catalytically competent form by phosphorylation of the activation loop within the kinase domain7: AMP binding to the γ-regulatory domain promotes phosphorylation by the upstream kinase8, protects the enzyme against dephosphorylation, as well as causing allosteric activation9. Here we show that ADP binding to just one of the two exchangeable AXP (AMP/ADP/ATP) binding sites on the regulatory domain protects the enzyme from dephosphorylation, although it does not lead to allosteric activation. Our studies show that active mammalian AMPK displays significantly tighter binding to ADP than to Mg-ATP, explaining how the enzyme is regulated under physiological conditions where the concentration of Mg-ATP is higher than that of ADP and much higher than that of AMP. We have determined the crystal structure of an active AMPK complex. The structure shows how the activation loop of the kinase domain is stabilized by the regulatory domain and how the kinase linker region interacts with the regulatory nucleotide-binding site that mediates protection against dephosphorylation. From our biochemical and structural data we develop a model for how the energy status of a cell regulates AMPK activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carling, D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem. Sci. 29, 18–24 (2004)
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Rev. Mol. Cell Biol. 8, 774–785 (2007)
Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15–25 (2005)
Shackelford, D. B. & Shaw, R. J. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Rev. Cancer 9, 563–575 (2009)
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006)
Huang, X. et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 412, 211–221 (2008)
Stein, S. C., Woods, A., Jones, N. A., Davison, M. D. & Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 345, 437–443 (2000)
Oakhill, J. S. et al. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl Acad. Sci. USA 107, 19237–19241 (2010)
Sanders, M. J., Grondin, P. O., Hegarty, B. D., Snowden, M. A. & Carling, D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403, 139–148 (2007)
Minokoshi, Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002)
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Med. 8, 1288–1295 (2002)
Watt, M. J. et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nature Med. 12, 541–548 (2006)
Andersson, U. et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279, 12005–12008 (2004)
Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004)
Hardie, D. G., Carling, D. & Sim, A. T. R. The AMP-activated protein kinase: a multisubstrate regulator of lipid metabolism. Trends Biochem. Sci. 14, 20–23 (1989)
Suter, M. et al. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 281, 32207–32216 (2006)
Carling, D., Sanders, M. J. & Woods, A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 32 (Suppl. 4). S55–S59 (2008)
Momcilovic, M., Hong, S. P. & Carlson, M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro . J. Biol. Chem. 281, 25336–25343 (2006)
Davies, S. P., Helps, N. R., Cohen, P. T. & Hardie, D. G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett. 377, 421–425 (1995)
Cheung, P. C., Salt, I. P., Davies, S. P., Hardie, D. G. & Carling, D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem. J. 346, 659–669 (2000)
Xiao, B. et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449, 496–500 (2007)
Kemp, B. E., Oakhill, J. S. & Scott, J. W. AMPK structure and regulation from three angles. Structure 15, 1161–1163 (2007)
Carling, D., Clarke, P. R., Zammit, V. A. & Hardie, D. G. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur. J. Biochem. 186, 129–136 (1989)
Veech, R. L., Lawson, J. W., Cornell, N. W. & Krebs, H. A. Cytosolic phosphorylation potential. J. Biol. Chem. 254, 6538–6547 (1979)
Hellsten, Y., Richter, E. A., Kiens, B. & Bangsbo, J. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J. Physiol. 520, 909–920 (1999)
McConell, G. K. et al. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J. Physiol. (Lond.) 568, 665–676 (2005)
Littler, D. R. et al. A conserved mechanism of autoinhibition for the AMPK kinase domain: ATP-binding site and catalytic loop refolding as a means of regulation. Acta Crystallogr. F 66, 143–151 (2010)
Otwinowski, Z. & Minor, W. Data Collection and Processing. Proc. CCP4 Study Weekend 556–562 (SERC, 1993)
Navaza, J. AMoRe: an Automated Package for Molecular Replacement. Acta Crystallogr. A 50, 157–163 (1994)
CCP4 . The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Acknowledgements
We thank M. Webb for gift of coumarin nucleotides, J. Skehel for comments on the manuscript and S. Smerdon for discussion and assistance. Work in both laboratories is supported by the MRC and we gratefully acknowledge Diamond Light Source for synchrotron access.
Author information
Authors and Affiliations
Contributions
B.X., M.J.S., E.U., R.H., F.V.M., D. Carmena, C.J., P.A.W., J.F.E., L.F.H., P.S., S.A.H., R.A. and S.R.M. performed experiments. All authors contributed to data analysis, experimental design and manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-12 with legends, Supplementary Tables 1-3 and Supplementary Methods which include 3 additional figures with legends. (PDF 2537 kb)
Rights and permissions
About this article
Cite this article
Xiao, B., Sanders, M., Underwood, E. et al. Structure of mammalian AMPK and its regulation by ADP. Nature 472, 230–233 (2011). https://doi.org/10.1038/nature09932
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09932