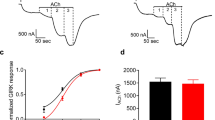
Abstract
Several neurotransmitters act through G-protein-coupled receptors to evoke a ‘slow’ excitation of neurons1,2. These include peptides, such as substance P and neurotensin, as well as acetylcholine and noradrenaline. Unlike the fast (approximately millisecond) ionotropic actions of small-molecule neurotransmitters, the slow excitation is not well understood at the molecular level, but can be mainly attributed to suppressing K+ currents and/or activating a non-selective cation channel3,4,5,6,7,8,9. The molecular identity of this cation channel has yet to be determined; similarly, how the channel is activated and its relative contribution to neuronal excitability induced by the neuropeptides are unknown. Here we show that, in the mouse hippocampal and ventral tegmental area neurons, substance P and neurotensin activate a channel complex containing NALCN and a large previously unknown protein UNC-80. The activation by substance P through TACR1 (a G-protein-coupled receptor for substance P) occurs by means of a unique mechanism: it does not require G-protein activation but is dependent on Src family kinases. These findings identify NALCN as the cation channel activated by substance P receptor, and suggest that UNC-80 and Src family kinases, rather than a G protein, are involved in the coupling from receptor to channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kandel, E. R., Schwartz, J. H. & Jessell, T. M. Principles of Neural Science 229–252 (McGraw-Hill, 2000)
Hille, B. Ion Channels of Excitable Membranes 201–236 (Sinauer, 2001)
Kuba, K. & Koketsu, K. Synaptic events in sympathetic ganglia. Prog. Neurobiol. 11, 77–169 (1978)
Jan, Y., Jan, L. & Kuffler, S. Further evidence for peptidergic transmission in smypathetic ganglia. Proc. Natl Acad. Sci. USA 77, 5008–5012 (1980)
Kuffler, S. & Sejnowski, T. Muscarinic and peptidergic excitation of bull-frog sympathetic neurons. J. Physiol. (Lond.) 341, 257–278 (1983)
Stanfield, P. R., Nakajima, Y. & Yamaguchi, K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature 315, 498–501 (1985)
Shen, K. Z. & North, R. A. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J. Physiol. (Lond.) 455, 471–485 (1992)
Shen, K. Z. & Surprenant, A. Common ionic mechanisms of excitation by substance P and other transmitters in guinea-pig submucosal neurones. J. Physiol. (Lond.) 462, 483–501 (1993)
Farkas, R. H., Chien, P. Y., Nakajima, S. & Nakajima, Y. Properties of a slow nonselective cation conductance modulated by neurotensin and other neurotransmitters in midbrain dopaminergic neurons. J. Neurophysiol. 76, 1968–1981 (1996)
Lu, B. et al. The neuronal NALCN channel contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129, 371–383 (2007)
Shen, K. Z. & North, R. A. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience 50, 345–353 (1992)
Aosaki, T. & Kawaguchi, Y. Actions of substance P on rat neostriatal neurons in vitro . J. Neurosci. 16, 5141–5153 (1996)
Inoue, K., Nakazawa, K., Inoue, K. & Fujimori, K. Nonselective cation channels coupled with tachykinin receptors in rat sensory neurons. J. Neurophysiol. 73, 736–742 (1995)
Pena, F. & Ramirez, J. M. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci. 24, 7549–7556 (2004)
Jones, S. W. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J. Physiol. (Lond.) 366, 63–87 (1985)
Otsuka, M. & Yoshioka, K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 73, 229–308 (1993)
Lefkowitz, R. J. & Shenoy, S. K. Transduction of receptor signals by β-arrestins. Science 308, 512–517 (2005)
DeFea, K. A. et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of β-arrestin-dependent scaffolding complex. Proc. Natl Acad. Sci. USA 97, 11086–11091 (2000)
Heuss, C., Scanziani, M., Gahwiler, B. H. & Gerber, U. G-protein-indenpendent signaling mediated by metabotropic glutamate receptors. Nature Neurosci. 2, 1070–1077 (1999)
Davis, M. J. et al. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 281, H1835–H1862 (2001)
Salters, M. W. & Kalia, L. V. Src kinases: a hub for NMDA receptor regulation. Nature Rev. Neurosci. 5, 317–328 (2004)
Heuss, C. & Gerber, U. G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci. 23, 469–475 (2000)
Montell, C., Birnbaumer, L. & Flockerzi, V. The TRP channels, a remarkably functional family. Cell 108, 595–598 (2002)
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003)
Oh, E. J., Gover, T. D., Cordoba-Rodriguez, R. & Weinreich, D. Substance P evokes cation currents through TRP channels in HEK293 cells. J. Neurophysiol. 90, 2069–2073 (2003)
Bley, K. R. & Tsien, R. W. Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron 4, 379–391 (1990)
Jospin, M. et al. UNC-80 and the NCA ion channels contribute to endocytosis defects in synaptojanin mutants. Curr. Biol. 17, 1595–1600 (2007)
Yeh, E. et al. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans . PLoS Biol. 6, e55 (2008)
Humphrey, J. A. et al. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr. Biol. 17, 624–629 (2007)
Liu, J., Xia, J., Cho, K. H., Clapham, D. E. & Ren, D. Catsper β: a novel transmembrane protein in the catsper channel complex. J. Biol. Chem. 282, 18945–18952 (2007)
Masuko, S., Nakajima, S. & Nakajima, Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience 49, 347–364 (1992)
Rayport, S. et al. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J. Neurosci. 12, 4264–4280 (1992)
Grace, A. A. & Onn, S.-P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci. 9, 3463–3481 (1989)
Acknowledgements
We thank D. Clapham, C. Deutsch, I. Medina, B. Novarro, M. Schmidt and H. Xu for critically reading earlier versions of the manuscript, J. Xia for help with experiments, H. Yu and L. Yue for cDNA constructs, and Sanofi-Aventis for the gift of SR48692. This work was supported, in part, by funding from American Heart Association, the NIH and the University of Pennsylvania Research Foundation.
Author Contributions B.L. did recordings from neurons (Figs 1–3 and Supplementary Fig. 2) and all the HEK293T cells (Fig. 4 and Supplementary Figs 1 and 7–10). Y.S. contributed to neuronal recordings (Figs 1 and 2 and Supplementary Figs 3 and 4). S.D. contributed to work in Fig. 2. H.W., Y.W. and J.L. did the protein work (Fig. 4 and Supplementary Fig. 6). D.R. started the project, designed experiments and developed the cDNA constructs. B.L. and D.R. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-10 with Legends (PDF 461 kb)
Rights and permissions
About this article
Cite this article
Lu, B., Su, Y., Das, S. et al. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457, 741–744 (2009). https://doi.org/10.1038/nature07579
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07579
This article is cited by
-
The NALCN channel regulates metastasis and nonmalignant cell dissemination
Nature Genetics (2022)
-
Structure of the human sodium leak channel NALCN in complex with FAM155A
Nature Communications (2020)
-
Intellectual disability-associated UNC80 mutations reveal inter-subunit interaction and dendritic function of the NALCN channel complex
Nature Communications (2020)
-
Structure of the human sodium leak channel NALCN
Nature (2020)
-
Functional expression of CLIFAHDD and IHPRF pathogenic variants of the NALCN channel in neuronal cells reveals both gain- and loss-of-function properties
Scientific Reports (2019)