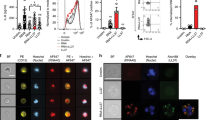
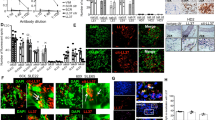
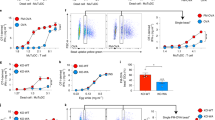
Abstract
Plasmacytoid dendritic cells (pDCs) sense viral and microbial DNA through endosomal Toll-like receptors to produce type 1 interferons. pDCs do not normally respond to self-DNA, but this restriction seems to break down in human autoimmune disease by an as yet poorly understood mechanism. Here we identify the antimicrobial peptide LL37 (also known as CAMP) as the key factor that mediates pDC activation in psoriasis, a common autoimmune disease of the skin. LL37 converts inert self-DNA into a potent trigger of interferon production by binding the DNA to form aggregated and condensed structures that are delivered to and retained within early endocytic compartments in pDCs to trigger Toll-like receptor 9. Thus, our data uncover a fundamental role of an endogenous antimicrobial peptide in breaking innate tolerance to self-DNA and suggest that this pathway may drive autoimmunity in psoriasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liu, Y. J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 (2005)
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nature Immunol. 5, 1219–1226 (2004)
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001)
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature Immunol. 5, 190–198 (2004)
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004)
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005)
Honda, K. et al. Spatiotemporal regulation of MyD88–IRF-7 signalling for robust type-I interferon induction. Nature 434, 1035–1040 (2005)
Krieg, A. M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002)
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006)
Stacey, K. J. et al. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J. Immunol. 170, 3614–3620 (2003)
Barton, G. M., Kagan, J. C. & Medzhitov, R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nature Immunol. 7, 49–56 (2006)
Yasuda, K. et al. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J. Immunol. 174, 6129–6136 (2005)
Ronnblom, L., Eloranta, M. L. & Alm, G. V. Role of natural interferon-α producing cells (plasmacytoid dendritic cells) in autoimmunity. Autoimmunity 36, 463–472 (2003)
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005)
Means, T. K. et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115, 407–417 (2005)
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005)
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001)
Lowes, M. A., Bowcock, A. M. & Krueger, J. G. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007)
Griffiths, C. E. & Voorhees, J. J. Psoriasis, T cells and autoimmunity. J. R. Soc. Med. 89, 315–319 (1996)
Nickoloff, B. J. & Nestle, F. O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664–1675 (2004)
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005)
Kupper, T. S. & Fuhlbrigge, R. C. Immune surveillance in the skin: mechanisms and clinical consequences. Nature Rev. Immunol. 4, 211–222 (2004)
Harder, J. & Schroder, J. M. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J. Leukoc. Biol. 77, 476–486 (2005)
Glaser, R. et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature Immunol. 6, 57–64 (2005)
Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75, 39–48 (2004)
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002)
Braff, M. H., Bardan, A., Nizet, V. & Gallo, R. L. Cutaneous defense mechanisms by antimicrobial peptides. J. Invest. Dermatol. 125, 9–13 (2005)
Ong, P. Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160 (2002)
Dufourcq, J., Neri, W. & Henry-Toulme, N. Molecular assembling of DNA with amphipathic peptides. FEBS Lett. 421, 7–11 (1998)
Niidome, T., Wakamatsu, M., Wada, A., Hirayama, T. & Aoyagi, H. Required structure of cationic peptide for oligonucleotide-binding and -delivering into cells. J. Pept. Sci. 6, 271–279 (2000)
Kubo, T. & Fujii, M. Specific binding and stabilization of DNA and phosphorothioate DNA by amphiphilic α-helical peptides. Nucleosides Nucleotides Nucleic Acids 20, 1313–1316 (2001)
Sandgren, S. et al. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J. Biol. Chem. 279, 17951–17956 (2004)
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000)
Guiducci, C. et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 203, 1999–2008 (2006)
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. A peptide antibiotic from human skin. Nature 387, 861 (1997)
Dorschner, R. A. et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus . J. Invest. Dermatol. 117, 91–97 (2001)
Heilborn, J. D. et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120, 379–389 (2003)
Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997)
Schauber, J. et al. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 18, 615–621 (2006)
Paulsen, F. et al. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J. Pathol. 198, 369–377 (2002)
Li, X., de Leeuw, E. & Lu, W. Total chemical synthesis of human psoriasin by native chemical ligation. Biochemistry 44, 14688–14694 (2005)
Zal, T., Zal, M. A., Lotz, C., Goergen, C. J. & Gascoigne, N. R. Spectral shift of fluorescent dye FM4-64 reveals distinct microenvironment of nuclear envelope in living cells. Traffic 7, 1607–1613 (2006)
Acknowledgements
We thank G. Lizee for critical reading of the manuscript; E. Wieder and K. Ramirez from the Flow Cytometry Core Facility for performing cell sorting; J. Wygant from the Monoclonal Antibodies Core Facility for generating the anti-LL37 monoclonal antibody; J. Bartels for ESI-MS analyses; and G. Reyes for technical assistance. This work was funded by a grant from the M. D. Anderson Cancer Foundation to M.G. Part of this work was supported by grants from the ‘Deutsche Forschungsgemeinschaft’ to J.-M.S. and to B.H.
Author Contributions R.L. performed and analysed most of the experiments in this study, and participated in their design. J.G. performed and analysed size-exclusion HPLC and helped in the atomic force microscopy experiments. V.F. and B.S. performed and analysed the experiments with transfected cell lines. B.C. and I.M. helped in establishing and analysing imaging experiments on endosomal trafficking. Yi-Hong Wang performed immunohistochemistry. B.H. collected and characterized clinical samples and participated in the expression analyses. W.C. generated the IRF7 construct. Yui-Hsi Wang helped in performing RT–PCR experiments. F.O.N. participated along with J.-M.S. and M.G. in the design of experiments with skin extracts. T.Z. helped in performing and evaluating imaging experiments using living cells. J.-M.S. designed, performed and evaluated the separation and biochemical analysis of skin extracts. Y.-J.L. provided critical suggestions and discussions throughout the study. M.G. conceived and supervised this study, was involved in the design and evaluation of all experiments, and wrote the manuscript with comments from co-authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
TITLE
This file contains Supplementary Figures S1-S7 and Legends. Figure S1 shows the identification of LL-37 in IFN-inducing HPLC fraction 26. Figure S2 shows the ability of genomic DNA of human and bacterial origin to induce IFN-α when coupled with LL-37. Figure S3 shows that the binding of LL-37 to DNA and the resulting structural changes in the DNA are reversible. Figure S4 shows internalization of LL-37 in the absence of DNA. Figure S5 shows that the ability of LL-37 to enhance DNA-mediated IFN-α requires the complex formation with the DNA. Figure S6 shows the preferential retention of LL-37-DNA in early endocytic compartments. Figure S7 shows the capacity of LL-37 to restore IFN-α induction by disrupted DNA aggregates. (PDF 1996 kb)
Rights and permissions
About this article
Cite this article
Lande, R., Gregorio, J., Facchinetti, V. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007). https://doi.org/10.1038/nature06116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06116
This article is cited by
-
Prediction of the synergistic effect of antimicrobial peptides and antimicrobial agents via supervised machine learning
BMC Biomedical Engineering (2024)
-
Cyclical palmitoylation regulates TLR9 signalling and systemic autoimmunity in mice
Nature Communications (2024)
-
Circulating cell-free DNA correlate to disease activity and treatment response of patients with radiographic axial spondyloarthritis
Scientific Reports (2024)
-
The Use of Microbial Modifying Therapies to Prevent Psoriasis Exacerbation and Associated Cardiovascular Comorbidity
Inflammation (2024)
-
Expression of molecular markers and synergistic anticancer effects of chemotherapy with antimicrobial peptides on glioblastoma cells
Cancer Chemotherapy and Pharmacology (2024)