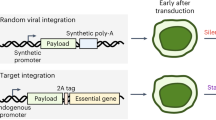
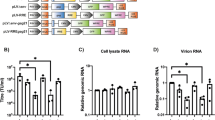
Abstract
Lentiviral vectors (LVs) offer the advantages of a large packaging capacity, broad cell tropism or specific cell-type targeting through pseudotyping, and long-term expression from integrated gene cassettes. However, transgene integration carries a risk of disrupting gene expression through insertional mutagenesis and may not be required for all applications. A non-integrating LV may be beneficial in cases in which transient gene expression is desired. Several recent publications outline the development and initial biological characterization of such vectors. Here, we discuss the potential applications and new directions for the development of integration-defective LVs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kumar M, Keller B, Makalou N, Sutton RE . Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther 2001; 12: 1893–1905.
Sinn PL, Sauter SL, McCray Jr PB . Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors—design, biosafety, and production. Gene Therapy 2005; 12: 1089–1098.
Leavitt AD, Shiue L, Varmus HE . Site-directed mutagenesis of HIV-1 integrase demonstrates differential effects on integrase functions in vitro. J Biol Chem 1993; 268: 2113–2119.
Leavitt AD, Robles G, Alesandro N, Varmus HE . Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol 1996; 70: 721–728.
Shibagaki Y, Holmes ML, Appa RS, Chow SA . Characterization of feline immunodeficiency virus integrase and analysis of functional domains. Virology 1997; 230: 1–10.
Shibagaki Y, Chow SA . Central core domain of retroviral integrase is responsible for target site selection. J Biol Chem 1997; 272: 8361–8369.
Talbott RL, Sparger EE, Lovelace KM, Fitch WM, Pedersen NC, Luciw PA et al. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA 1989; 86: 5743–5747.
Nisole S, Saib A . Early steps of retrovirus replicative cycle. Retrovirology 2004; 1: 9.
Craigie R, Fujiwara T, Bushman F . The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell 1990; 62: 829–837.
Katz RA, Merkel G, Kulkosky J, Leis J, Skalka AM . The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell 1990; 63: 87–95.
Katz RA, Skalka AM . The retroviral enzymes. Annu Rev Biochem 1994; 63: 133–173.
Engelman A, Craigie R . Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol 1992; 66: 6361–6369.
Nightingale SJ, Hollis RP, Pepper KA, Petersen D, Yu XJ, Yang C et al. Transient gene expression by nonintegrating lentiviral vectors. Mol Ther 2006; 13: 1121–1132.
Mizuuchi K . Polynucleotidyl transfer reactions in transpositional DNA recombination. J Biol Chem 1992; 267: 21273–21276.
Goff SP . Retroviridae: the retroviruses and their replication. In: David M Knipe PMH, Diane E Griffin, Malcolm A Martin, Robert A Lamb, Bernard Roizman, Stephen E Strauss (ed). Fields Virology, 4th edn, vol. 2. Lippincott Williams & Wilkins: Philadelphia, 2001. pp 1871–1940.
Yoder KE, Bushman FD . Repair of gaps in retroviral DNA integration intermediates. J Virol 2000; 74: 11191–11200.
Farnet CM, Haseltine WA . Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol 1991; 65: 6942–6952.
Pauza CD, Trivedi P, McKechnie TS, Richman DD, Graziano FM . 2-LTR circular viral DNA as a marker for human immunodeficiency virus type 1 infection in vivo. Virology 1994; 205: 470–478.
Pages JC, Bru T . Toolbox for retrovectorologists. J Gene Med 2004; 6 (Suppl 1): S67–S82.
Miller MD, Wang B, Bushman FD . Human immunodeficiency virus type 1 preintegration complexes containing discontinuous plus strands are competent to integrate in vitro. J Virol 1995; 69: 3938–3944.
Li L, Olvera JM, Yoder KE, Mitchell RS, Butler SL, Lieber M et al. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J 2001; 20: 3272–3281.
Bayer M, Kantor B, Cockrell A, Ma H, Zeithaml B, Li X et al. A large U3 deletion causes increased in vivo expression from a nonintegrating lentiviral vector. Mol Ther 2008; 16: 1968–1976.
Butler SL, Johnson EP, Bushman FD . Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J Virol 2002; 76: 3739–3747.
Nandi JS . Unintegrated viral DNA as a marker for human immunodeficiency virus 1 infection in vivo and in vitro. Acta Virol 1999; 43: 367–372.
Brussel A, Sonigo P . Evidence for gene expression by unintegrated human immunodeficiency virus type 1 DNA species. J Virol 2004; 78: 11263–11271.
Stevenson M, Haggerty S, Lamonica CA, Meier CM, Welch SK, Wasiak AJ . Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J Virol 1990; 64: 2421–2425.
Shoemaker C, Hoffman J, Goff SP, Baltimore D . Intramolecular integration within Moloney murine leukemia virus DNA. J Virol 1981; 40: 164–172.
Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF . Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J Virol 2002; 76: 4138–4144.
Barquinero J, Eixarch H, Perez-Melgosa M . Retroviral vectors: new applications for an old tool. Gene Therapy 2004; 11 (Suppl 1): S3–S9.
Baum C, Schambach A, Bohne J, Galla M . Retrovirus vectors: toward the plentivirus? Mol Ther 2006; 13: 1050–1063.
Rodrigues T, Carrondo MJ, Alves PM, Cruz PE . Purification of retroviral vectors for clinical application: biological implications and technological challenges. J Biotechnol 2007; 127: 520–541.
Wu X, Li Y, Crise B, Burgess SM . Transcription start regions in the human genome are favored targets for MLV integration. Science 2003; 300: 1749–1751.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest 2008; 118: 3143–3150.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 2008; 118: 3132–3142.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2004; 2: E234.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F . HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002; 110: 521–529.
Wang GP, Ciuffi A, Leipzig J, Berry CC, Bushman FD . HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res 2007; 17: 1186–1194.
Kang Y, Moressi CJ, Scheetz TE, Xie L, Tran DT, Casavant TL et al. Integration site choice of a feline immunodeficiency virus vector. J Virol 2006; 80: 8820–8823.
Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 2009; 119: 964–975.
Metais JY, Dunbar CE . The MDS1-EVI1 gene complex as a retrovirus integration site: impact on behavior of hematopoietic cells and implications for gene therapy. Mol Ther 2008; 16: 439–449.
Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 2007; 25: 1298–1306.
Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M et al. Rapid ‘open-source’ engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 2008; 31: 294–301.
Yu SS, Dan K, Chono H, Chatani E, Mineno J, Kato I . Transient gene expression mediated by integrase-defective retroviral vectors. Biochem Biophys Res Commun 2008; 368: 942–947.
Philippe S, Sarkis C, Barkats M, Mammeri H, Ladroue C, Petit C et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc Natl Acad Sci USA 2006; 103: 17684–17689.
Saenz DT, Loewen N, Peretz M, Whitwam T, Barraza R, Howell KG et al. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J Virol 2004; 78: 2906–2920.
Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 2006; 12: 348–353.
Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther 2007; 15: 1947–1954.
Cornu TI, Cathomen T . Targeted genome modifications using integrase-deficient lentiviral vectors. Mol Ther 2007; 15: 2107–2113.
Coutant F, Frenkiel MP, Despres P, Charneau P . Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One 2008; 3: e3973.
Moldt B, Staunstrup NH, Jakobsen M, Yanez-Munoz RJ, Mikkelsen JG . Genomic insertion of lentiviral DNA circles directed by the yeast Flp recombinase. BMC Biotechnol 2008; 8: 60.
Negri DR, Michelini Z, Baroncelli S, Spada M, Vendetti S, Buffa V et al. Successful immunization with a single injection of non-integrating lentiviral vector. Mol Ther 2007; 15: 1716–1723.
Okada Y, Ueshin Y, Hasuwa H, Takumi K, Okabe M, Ikawa M . Targeted gene modification in mouse ES cells using integrase-defective lentiviral vectors. Genesis 2009; 47: 217–223.
Rahim AA, Wong AM, Howe SJ, Buckley SM, Acosta-Saltos AD, Elston KE et al. Efficient gene delivery to the adult and fetal CNS using pseudotyped non-integrating lentiviral vectors. Gene Therapy 2009; 16: 509–520.
Staunstrup NH, Moldt B, Mates L, Villesen P, Jakobsen M, Ivics Z et al. Hybrid lentivirus-transposon vectors with a random integration profile in human cells. Mol Ther 2009; 17: 1505–1514.
Vargas Jr J, Gusella GL, Najfeld V, Klotman ME, Cara A . Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum Gene Ther 2004; 15: 361–372.
Vargas Jr J, Klotman ME, Cara A . Conditionally replicating lentiviral-hybrid episomal vectors for suicide gene therapy. Antiviral Res 2008; 80: 288–294.
Vink CA, Gaspar HB, Gabriel R, Schmidt M, McIvor RS, Thrasher AJ et al. Sleeping beauty transposition from nonintegrating lentivirus. Mol Ther 2009; 17: 1197–1204.
Loewen N, Leske DA, Chen Y, Teo WL, Saenz DT, Peretz M et al. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J Gene Med 2003; 5: 1009–1017.
van Gent DC, Oude Groeneger AA, Plasterk RH . Identification of amino acids in HIV-2 integrase involved in site-specific hydrolysis and alcoholysis of viral DNA termini. Nucleic Acids Res 1993; 21: 3373–3377.
Khan E, Mack JP, Katz RA, Kulkosky J, Skalka AM . Retroviral integrase domains: DNA binding and the recognition of LTR sequences. Nucleic Acids Res 1991; 19: 851–860.
Ellison V, Brown PO . A stable complex between integrase and viral DNA ends mediates human immunodeficiency virus integration in vitro. Proc Natl Acad Sci USA 1994; 91: 7316–7320.
Engelman A, Bushman FD, Craigie R . Identification of discrete functional domains of HIV-1 integrase and their organization within an active multimeric complex. EMBO J 1993; 12: 3269–3275.
Ellison V, Gerton J, Vincent KA, Brown PO . An essential interaction between distinct domains of HIV-1 integrase mediates assembly of the active multimer. J Biol Chem 1995; 270: 3320–3326.
Craigie R . HIV integrase, a brief overview from chemistry to therapeutics. J Biol Chem 2001; 276: 23213–23216.
Lu R, Limon A, Ghory HZ, Engelman A . Genetic analyses of DNA-binding mutants in the catalytic core domain of human immunodeficiency virus type 1 integrase. J Virol 2005; 79: 2493–2505.
Engelman A, Hickman AB, Craigie R . The core and carboxyl-terminal domains of the integrase protein of human immunodeficiency virus type 1 each contribute to nonspecific DNA binding. J Virol 1994; 68: 5911–5917.
Kulkosky J, Jones KS, Katz RA, Mack JP, Skalka AM . Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol Cell Biol 1992; 12: 2331–2338.
Drelich M, Wilhelm R, Mous J . Identification of amino acid residues critical for endonuclease and integration activities of HIV-1 IN protein in vitro. Virology 1992; 188: 459–468.
Wiskerchen M, Muesing MA . Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J Virol 1995; 69: 376–386.
Lutzke RA, Plasterk RH . Structure-based mutational analysis of the C-terminal DNA-binding domain of human immunodeficiency virus type 1 integrase: critical residues for protein oligomerization and DNA binding. J Virol 1998; 72: 4841–4848.
Naldini L, Blomer U, Gage FH, Trono D, Verma IM . Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996; 93: 11382–11388.
Park F, Ohashi K, Kay MA . Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood 2000; 96: 1173–1176.
Wu Y . HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology 2004; 1: 13.
Nakajima N, Lu R, Engelman A . Human immunodeficiency virus type 1 replication in the absence of integrase-mediated dna recombination: definition of permissive and nonpermissive T-cell lines. J Virol 2001; 75: 7944–7955.
Gaur M, Leavitt AD . Mutations in the human immunodeficiency virus type 1 integrase D,D(35)E motif do not eliminate provirus formation. J Virol 1998; 72: 4678–4685.
Lim JK, Glass WG, McDermott DH, Murphy PM . CCR5: no longer a ‘good for nothing’ gene—chemokine control of West Nile virus infection. Trends Immunol 2006; 27: 308–312.
Lombardo A, Cesana D, Genovese P, Provasi E, Maruggi G, Binini C et al. Characterization of potential genomic ‘safe harbor’ for efficient targeted gene addition with zinc finger nucleases. Mol Ther 2009; 17: S168.
Mairhofer J, Grabherr R . Rational vector design for efficient non-viral gene delivery: challenges facing the use of plasmid DNA. Mol Biotechnol 2008; 39: 97–104.
Chen ZY, He CY, Meuse L, Kay MA . Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Therapy 2004; 11: 856–864.
Mayrhofer P, Blaesen M, Schleef M, Jechlinger W . Minicircle-DNA production by site specific recombination and protein-DNA interaction chromatography. J Gene Med 2008; 10: 1253–1269.
Davidson BL, Boudreau RL . RNA interference: a tool for querying nervous system function and an emerging therapy. Neuron 2007; 53: 781–788.
Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J . Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA 2004; 101: 8676–8681.
Snove Jr O, Rossi JJ . Expressing short hairpin RNAs in vivo. Nat Methods 2006; 3: 689–695.
Scherr M, Eder M . Gene silencing by small regulatory RNAs in mammalian cells. Cell Cycle 2007; 6: 444–449.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banasik, M., McCray, P. Integrase-defective lentiviral vectors: progress and applications. Gene Ther 17, 150–157 (2010). https://doi.org/10.1038/gt.2009.135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.135
Keywords
This article is cited by
-
Stable expression of large transgenes via the knock-in of an integrase-deficient lentivirus
Nature Biomedical Engineering (2023)
-
Homology-directed gene-editing approaches for hematopoietic stem and progenitor cell gene therapy
Stem Cell Research & Therapy (2021)
-
Engineering of α-PD-1 antibody-expressing long-lived plasma cells by CRISPR/Cas9-mediated targeted gene integration
Cell Death & Disease (2020)
-
LV305, a dendritic cell-targeting integration-deficient ZVex TM -based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response
Molecular Therapy - Oncolytics (2016)
-
Non-integrating lentiviral vectors based on the minimal S/MAR sequence retain transgene expression in dividing cells
Science China Life Sciences (2016)