Abstract
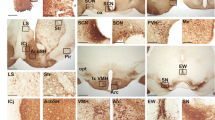
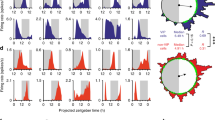
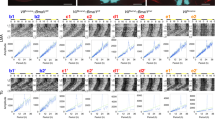
The suprachiasmatic nucleus (SCN) controls the circadian rhythm of physiological and behavioural processes in mammals. Here we show that prokineticin 2 (PK2), a cysteine-rich secreted protein, functions as an output molecule from the SCN circadian clock. PK2 messenger RNA is rhythmically expressed in the SCN, and the phase of PK2 rhythm is responsive to light entrainment. Molecular and genetic studies have revealed that PK2 is a gene that is controlled by a circadian clock (clock-controlled). Receptor for PK2 (PKR2) is abundantly expressed in major target nuclei of the SCN output pathway. Inhibition of nocturnal locomotor activity in rats by intracerebroventricular delivery of recombinant PK2 during subjective night, when the endogenous PK2 mRNA level is low, further supports the hypothesis that PK2 is an output molecule that transmits behavioural circadian rhythm. The high expression of PKR2 mRNA within the SCN and the positive feedback of PK2 on its own transcription through activation of PKR2 suggest that PK2 may also function locally within the SCN to synchronize output.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klein, D. C., Moore, R. Y. & Reppert, S. M. (eds) Suprachiasmatic Nucleus: The Mind's Clock 467 (Oxford Univ. Press, New York, 1991)
Moore, R. Y. Circadian rhythms: basic neurobiology and clinical applications. Ann. Rev. Med. 48, 253–266 (1997)
Dunlap, J. C. Molecular bases for circadian clocks. Cell 96, 271–290 (1999)
Gillette, M. U. & Tischkau, S. A. Suprachiasmatic nucleus: the brain's clock. Rec. Prog. Hormone Res. 54, 33–59 (1999)
Hastings, M. H. Circadian clockwork: two loops are better than one. Nature Rev. Neurosci. 1, 143–146 (2000)
Reppert, S. M. & Weaver, D. R. Molecular analysis of mammalian circadian rhythms. Ann. Rev. Physiol. 63, 647–676 (2001)
Allada, R., Emery, P., Takahashi, J. S. & Rosbash, M. Stopping time: the genetics of fly and mouse circadian clocks. Ann. Rev. Neurosci. 24, 1091–1119 (2001)
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998)
Hogenesch, J. B., Gu, Y. Z., Jain, S. & Bradfield, C. A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl Acad. Sci. USA 95, 5474–5479 (1998)
Kume, K. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 (1999)
Shearman, L. P. et al. Interacting molecular loops in the mammalian circadian clock. Science 288, 917–924 (2000)
Vitaterna, M. H. et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl Acad. Sci. USA 96, 12114–12119 (1999)
Thresher, R. J. et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998)
Shearman, L. P., Jin, X., Lee, C., Reppert, S. M. & Weaver, D. R. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol. Cell Biol. 20, 6269–6275 (2000)
van der Horst, G. T. J. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999)
Cermakian, N., Monaco, L., Pando, M. P., Dierich, A. & Sassone-Corsi, P. Altered behavioural rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 20, 3967–3974 (2001)
Vitaterna, M. H. et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behaviour. Science 264, 719–725 (1994)
King, D. P. et al. Positional cloning of the mouse circadian Clock gene. Cell 89, 641–653 (1997)
Bae, K. et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30, 525–536 (2001)
Zheng, B. et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 (2001)
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000)
Lowrey, P. L. et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–491 (2000)
Hardin, P. E. From biological clock to biological rhythms. Genome Biol. 1, 1023.1–1023.5 (2000)
Renn, S. C., Park, J. H., Rosbash, M., Hall, J. C. & Taghert, P. H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioural circadian rhythms in Drosophila. Cell 99, 791–802 (1999)
Sarov-Blat, L., So, W. V., Liu, L. & Rosbash, M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behaviour. Cell 101, 647–656 (2000)
Ralph, M. R., Foster, R. G., Davis, F. C. & Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 (1990)
Silver, R., LeSauter, J., Tresco, P. A. & Lehman, M. N. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813 (1996)
Earnest, D. J., Liang, F. Q., Ratcliff, M. & Cassone, V. M. Immortal time: circadian clock properties of rat suprachiasmatic cell lines. Science 283, 693–695 (1996)
Jin, X. et al. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96, 57–68 (1999)
Kalsbeek, A., van Heerikhuize, J. J., Wortel, J. & Buijs, R. M. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J. Neurosci. 16, 5555–5565 (1996)
Boer, G. J., van Esseveldt, K. E., van der Geest, B. A., Duindam, H. & Reitveld, W. J. Vasopressin-deficient suprachiasmatic nucleus grafts reinstate circadian rhythmicity in suprachiasmatic nucleus-lesioned arrhthmic rats. Neuroscience 89, 375–385 (1999)
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signalling. Science 294, 2511–2515 (2001)
Lopez-Molina, L., Conquet, F., Dubois-Dauphin, M. & Schibler, U. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behaviour. EMBO J. 16, 6762–6771 (1997)
Li, M., Bullock, C. M., Knauer, D. J., Ehlert, F. J. & Zhou, Q. Y. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 59, 692–698 (2001)
Jilek, A., Engel, E., Beier, D. & Lepperdinger, G. Murine Bv8 gene maps near a synteny breakpoint of mouse chromosome 6 and human 3p21. Gene 256, 189–195 (2000)
Okamura, H. et al. Photic induction of mPer1 and mPer2 in Cry-deficient mice lacking a biological clock. Science 286, 2531–2534 (1999)
Wilsbacher, L. D. et al. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice in vivo. Proc. Natl Acad. Sci. USA 99, 489–494 (2002)
Daan, S. & Pittendrigh, C. S. A funtional abalysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 106, 253–266 (1976)
Pittendrigh, C. S. Temporal organization: reflections of a Darwinian clock-watcher. Ann. Rev. Physiol. 55, 16–54 (1993)
Roenneberg, T. & Foster, R. G. Twilight times: light and the circadian system. Photochem. Photobiol. 66, 549–561 (1997)
Watts, A. G., Swanson, L. W. & Sanchez-Watts, G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 258, 204–229 (1987)
Watts, A. G. & Swanson, L. W. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 258, 230–252 (1987)
Leak, R. K. & Moore, R. Y. Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol. 433, 312–334 (2001)
Buijs, R. M. The anatomical basis for the expression of circadian rhythms: the efferent projections of the suprachiasmatic nucleus. Prog. Brain Res. 111, 229–240 (1996)
Albrecht, U., Sun, Z. S., Eichele, G. & Lee, C. C. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91, 1055–1064 (1997)
Tei, H. et al. Circadian oscillation of a mammalian homolog of the Drosophila period gene. Nature 389, 512–516 (1997)
Miyamoto, Y. & Sancar, A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl Acad. Sci. USA 95, 6097–6102 (1998)
Zylka, M. J., Shearman, L. P., Weaver, D. R. & Reppert, S. M. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20, 1103–1110 (1998)
LeSauter, J. & Silver, R. Output signals of the SCN. Chronobiol. Int. 15, 535–550 (1998)
Winzer-Serhan, U. H., Broide, R. S., Chen, Y. & Leslie, F. M. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res. Brain Res. Protocols 3, 229–241 (1999)
Acknowledgements
We thank Y. Chen, D. Lin, H. Nagasaki and L. Shearman for technical assistance; S. Loughlin, S. Reppert and P. Sassone-Corsi for discussions; and B. Semler for access to a luminometer. We also thank J. Takahashi, M. Vitaterna and B. van der Horst for providing access to mutant mice. The research is partially supported by grants from the National Institutes of Health. C.M.B. is a recipient of a UC Irvine MSTP training grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cheng, M., Bullock, C., Li, C. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 (2002). https://doi.org/10.1038/417405a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/417405a
This article is cited by
-
In vivo recording of the circadian calcium rhythm in Prokineticin 2 neurons of the suprachiasmatic nucleus
Scientific Reports (2023)
-
A new phase model of the spatiotemporal relationships between three circadian oscillators in the brainstem
Scientific Reports (2023)
-
A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus
Nature Communications (2023)
-
Cystic Fibrosis and Sleep Circadian Rhythms
Pulmonary Therapy (2022)
-
Molecular screening of PROKR2 gene in girls with idiopathic central precocious puberty
Italian Journal of Pediatrics (2021)