Abstract
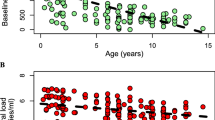
The thymus represents the major site of the production and generation of T cells expressing αβ-type T-cell antigen receptors1. Age-related involution2 may affect the ability of the thymus to reconstitute T cells expressing CD4 cell-surface antigens that are lost during HIV infection3; this effect has been seen after chemotherapy and bone-marrow transplantation4,5. Adult HIV-infected patients treated with highly active antiretroviral therapy (HAART) show a progressive increase in their number of naive CD4-positive T cells6,7. These cells could arise through expansion of existing naive T cells in the periphery8 or through thymic production of new naive T cells9,10. Here we quantify thymic output by measuring the excisional DNA products of TCR-gene rearrangement. We find that, although thymic function declines with age, substantial output is maintained into late adulthood. HIV infection leads to a decrease in thymic function that can be measured in the peripheral blood and lymphoid tissues. In adults treated with HAART, there is a rapid and sustained increase in thymic output in most subjects. These results indicate that the adult thymus can contribute to immune reconstitution following HAART.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Picker, L. & Siegelman, M. in Fundamental Immunology (ed. Paul, W. E.) 145–197 (Raven, New York, 1993).
Steinmann, G. Changes in the human thymus during aging. Curr. Top. Pathol. 75, 43–80 (1986).
Pantaleo, G., Graziosi, C. & Fauci, A. The immunopathogenesis of human immunodeficiency virus infection. New Engl. J. Med. 328, 327–335 (1993).
Mackall, C. & Gress, R. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol. Rev. 157, 61–72 (1997).
Mackall, C.et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. New Engl. J. Med. 332, 143–149 (1995).
Autran, B.et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277, 112–116 (1997).
Zhang, Z.-Q.et al. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc. Natl Acad. Sci. USA 95, 1154–1159 (1998).
Haynes, B.et al. Analysis of the role of the adult thymus in reconstitution of peripheral T lymphocytes in human immunodeficiency virus type 1 infection. J. Clin. Invest. (submitted).
McCune, J.et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J. Clin. Invest. 101, 2301–2308 (1998).
Dybul, M., Kinter, A., Ruiz, M. & Fauci, A. Promethean thymus? J. Clin. Invest. 101, 2299–2300 (1998).
Kong, F.-K., Chen, C.-L. & Cooper, M. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity 8, 97–104 (1998).
Livak, F. & Schatz, D. T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 16, 609–618 (1996).
Takeshita, S., Toda, M. & Ymagishi, H. Excision products of the T cell receptor gene support a progressive rearrangement model of the α/δ locus. EMBO J. 8, 3261–3270 (1989).
Bogue, M. & Roth, D. B. Mechanism of V(D)J recombination. Curr. Opin. Immunol. 8, 175–180 (1996).
Verschuren, M.et al. Preferential rearrangements of the T cell receptor-δ-deleting elements in human T cells. J. Immunol. 158, 1208–1216 (1997).
Picker, L.et al. Control of lymphocyte recirculation in man. J. Immunol. 150, 1105–1121 (1993).
Petrie, H., Livak, F., Burtrum, D. & Mazel, S. Tcell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J. Exp. Med. 182, 121–127 (1995).
Piatak, M. Jet al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259, 1749–1754 (1993).
Cossarizza, A.et al. Age-related imbalance of virgin (CD45RA+) and memory (CD45R0+) cells between CD4+ and CD8+ T lymphocytes in humans: a study from newborns to centenarians. J. Immunol. Res. 4, 118–126 (1992).
McLean, A. & Michie, C. In vivo estimates of division and death rates of human T lymphocytes. Proc. Natl Acad. Sci. USA 92, 3707–3711 (1995).
Schnittman, S.et al. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl Acad. Sci. USA 87, 6058–6062 (1990).
Chun, T., Chadwick, K., Margolick, J. & Siliciano, R. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J. Virol. 71, 4436–4444 (1997).
Schnittman, S. M.et al. Evidence for susceptibility of intrathymic T cell precursors to human immunodeficiency virus infection: a mechanism for T4 (CD4) lymphocyte depletion. Trans. Assoc. Am. Physicians 103, 96–101 (1990).
Wykrzykowska, J.et al. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J. Exp. Med. 187, 1767–1778 (1998).
Bonyhadi, M.et al. HIV induces thymus depletion in vivo. Nature 363, 728–732 (1993).
Aldrovandi, G. M.et al. The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 (1993).
Pakker, N.et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nature Med. 4, 208–214 (1998).
Gorochov, G.et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nature Med. 4, 215–221 (1998).
Withers-Ward, E.et al. Transient reneweal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nature Med. 3, 1102–1109 (1997).
Han, S.et al. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science 278, 301–305 (1997).
Acknowledgements
We thank J. Wong for monoclonal antibody 12F6; M. Gately for rhIL-2; F. Scott, J. McKinsey, A. Rahimi, S. Norris, G. Sempowski, J. Tomasch and M. Zupancic for sample collection and processing; A. Mobley and B. Darnell for FACS support; B. Dawson for viral load measurement; R.Scheuermann and D. Sodora for advice; and all the study participants for their cooperation. This work was supported by grants from the NIH and the American Foundation for AIDS Research. R.A.K., M.B.F. and J.A.Z. are Elizabeth Glaser Scientists of the Pediatric AIDS Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douek, D., McFarland, R., Keiser, P. et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 396, 690–695 (1998). https://doi.org/10.1038/25374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25374
This article is cited by
-
Role of thymosin α1 in restoring immune response in immunological nonresponders living with HIV
BMC Infectious Diseases (2024)
-
Prolonged experimental CD4+ T-cell depletion does not cause disease progression in SIV-infected African green monkeys
Nature Communications (2023)
-
Blood levels of T-Cell Receptor Excision Circles (TRECs) provide an index of exposure to traumatic stress in mice and humans
Translational Psychiatry (2022)
-
Protective reactive thymus hyperplasia in COVID-19 acute respiratory distress syndrome
Critical Care (2021)
-
Age-dependency of terminal ileum tissue resident memory T cell responsiveness profiles to S. Typhi following oral Ty21a immunization in humans
Immunity & Ageing (2021)