Abstract
Purpose of Review
GBA mutations are the most common known genetic cause of Parkinson’s disease (PD). Its biological pathway may be important in idiopathic PD, since activity of the enzyme encoded by GBA, glucocerebrosidase, is reduced even among PD patients without GBA mutations. This article describes the structure and function of GBA, reviews recent literature on the clinical phenotype of GBA PD, and suggests future directions for research, counseling, and treatment.
Recent Findings
Several longitudinal studies have shown that GBA PD has faster motor and cognitive progression than idiopathic PD and that this effect is dose dependent. New evidence suggests that GBA mutations may be important in multiple system atrophy. Further, new interventional studies focusing on GBA PD are described. These studies may increase the interest of PD patients and caregivers in genetic counseling.
Summary
GBA mutation status may help clinicians estimate PD progression, though mechanisms underlying GBA and synucleinopathy require further understanding.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89(9):691–4.
Van Bogaert L, Froehlich A. Un cas de maladie de Gaucher de l'adulte avec syndrome de Raynaud, pigmentation, et rigidite du type extrapyrajidal aux membres inferieurs. Ann Med. 1939;45:57–70.
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–7.
Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–83.
Zimran A, Neudorfer O, Elstein D. The glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2005;352(7):728–31. author reply 728-31
•• Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, Mirelman A, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84(9):880–7. This meta-analysis showed differential odds ratios for PD between mild and severe GBA mutation carriers (2.2 and 10.3, respectively), as well as differential age of PD onset, with severe GBA mutation carriers having earlier age of onset than mild GBA mutation carriers (53.1 vs. 58.1, respectively).
Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Ross BM, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116–22.
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361(17):1651–61.
Bras J, Paisan-Ruiz C, Guerreiro R, Ribeiro MH, Morgadinho A, Januario C, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2009;30(9):1515–7.
Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66(5):578–83.
Clark LN, Nicolai A, Afridi S, Harris J, Mejia-Santana H, Strug L, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson's disease in subjects of Jewish ethnicity. Mov Disord. 2005;20(1):100–3.
Clark LN, Ross BM, Wang Y, Mejia-Santana H, Harris J, Louis ED, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69(12):1270–7.
De Marco EV, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in southern Italy. Mov Disord. 2008;23(3):460–3.
Eblan MJ, Nguyen J, Ziegler SG, Lwin A, Hanson M, Gallardo M, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21(2):282–3.
Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120(5):641–9.
Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452(2):87–9.
Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65(3):379–82.
Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66(5):571–6.
Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132(Pt 7):1783–94.
Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72(4):310–6.
Sato C, Morgan A, Lang AE, Salehi-Rad S, Kawarai T, Meng Y, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord. 2005;20(3):367–70.
Emelyanov A, Boukina T, Yakimovskii A, Usenko T, Drosdova A, Zakharchuk A, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in Russia. Mov Disord. 2012;27(1):158–9.
Huang CL, Wu-Chou YH, Lai SC, Chang HC, Yeh TH, Weng YH, et al. Contribution of glucocerebrosidase mutation in a large cohort of sporadic Parkinson's disease in Taiwan. Eur J Neurol. 2011;18(10):1227–32.
Lesage S, Condroyer C, Hecham N, Anheim M, Belarbi S, Lohman E, et al. Mutations in the glucocerebrosidase gene confer a risk for Parkinson disease in North Africa. Neurology. 2011;76(3):301–3.
Moraitou M, et al. beta-Glucocerebrosidase gene mutations in two cohorts of Greek patients with sporadic Parkinson's disease. Mol Genet Metab. 2011;104(1–2):149–52.
Socal MP, Bock H, Michelin-Tirelli K, Hilbig A, Saraiva-Pereira ML, Rieder CRM, et al. Parkinson's disease and the heterozygous state for glucocerebrosidase mutations among Brazilians. Parkinsonism Relat Disord. 2009;15(1):76–8.
Spitz M, Rozenberg R, da Veiga Pereira L, Reis Barbosa E. Association between Parkinson's disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14(1):58–62.
Tan EK, Tong J, Fook-Chong S, Yih Y, Wong MC, Pavanni R, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64(7):1056–8.
Wu YR, Chen CM, Chao CY, Ro LS, Lyu RK, Chang KH, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78(9):977–9.
Ziegler SG, Eblan MJ, Gutti U, Hruska KS, Stubblefield BK, Goker-Alpan O, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab. 2007;91(2):195–200.
Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. 2017;49(10):1511–6.
Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. 2014;46(9):989–93.
Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA, et al. Meta-analysis of Parkinson disease: identification of a novel locus, RIT2. Ann Neurol. 2012;71(3):370–84.
Sidransky E. Gaucher disease: complexity in a "simple" disorder. Mol Genet Metab. 2004;83(1–2):6–15.
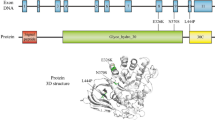
Reiner O, Wigderson M, Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988;7(2):107–16.
Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4(1):87–96.
Martinez-Arias R, et al. Sequence variability of a human pseudogene. Genome Res. 2001;11(6):1071–85.
Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. Analyses of variant acid beta-glucosidases: effects of Gaucher disease mutations. J Biol Chem. 2006;281(7):4242–53.
Sorge JA, West C, Kuhl W, Treger L, Beutler E. The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet. 1987;41(6):1016–24.
Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29(5):567–83.
Pasmanik-Chor M, et al. Overexpression of human glucocerebrosidase containing different-sized leaders. Biochem J. 1996;317(Pt 1):81–8.
Van Weely S, et al. Function of oligosaccharide modification in glucocerebrosidase, a membrane-associated lysosomal hydrolase. Eur J Biochem. 1990;191(3):669–77.
Aerts JM, et al. Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim Biophys Acta Gen Subj. 1988;964(3):303–8.
Dierks T, Schlotawa L, Frese MA, Radhakrishnan K, von Figura K, Schmidt B. Molecular basis of multiple sulfatase deficiency, mucolipidosis II/III and Niemann-pick C1 disease - lysosomal storage disorders caused by defects of non-lysosomal proteins. Biochim Biophys Acta. 2009;1793(4):710–25.
Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–83.
Erickson AH, Ginns EI, Barranger JA. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985;260(26):14319–24.
Imai K. Characterization of beta-glucosidase as a peripheral enzyme of lysosomal membranes from mouse liver and purification. J Biochem. 1985;98(5):1405–16.
Morimoto S, Kishimoto Y, Tomich J, Weiler S, Ohashi T, Barranger JA, et al. Interaction of saposins, acidic lipids, and glucosylceramidase. J Biol Chem. 1990;265(4):1933–7.
Vaccaro AM, Tatti M, Ciaffoni F, Salvioli R, Maras B, Barca A. Function of saposin C in the reconstitution of glucosylceramidase by phosphatidylserine liposomes. FEBS Lett. 1993;336(1):159–62.
Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, et al. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4(7):704–9.
Beutler E, Gelbart T, Scott CR. Hematologically important mutations: Gaucher disease. Blood Cells Mol Dis. 2005;35(3):355–64.
Montfort M, Chabás A, Vilageliu L, Grinberg D. Functional analysis of 13 GBA mutant alleles identified in Gaucher disease patients: pathogenic changes and "modifier" polymorphisms. Hum Mutat. 2004;23(6):567–75.
• Alcalay RN, Levy OA, Waters CH, Fahn S, Ford B, Kuo SH, et al. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain. 2015;138(Pt 9):2648–58. The authors show that GCase activity was significantly different between PD patients and controls, even among non-carriers of GBA or LRRK2 mutations, suggesting that the biological pathway of GBA is important to idiopathic PD. Further, higher GCase activity was associated with longer disease duration, suggesting a milder disease course.
Zimmer KP, le Coutre P, Aerts HMFG, Harzer K, Fukuda M, O'Brien JS, et al. Intracellular transport of acid beta-glucosidase and lysosome-associated membrane proteins is affected in Gaucher's disease (G202R mutation). J Pathol. 1999;188(4):407–14.
Schmitz M, Alfalah M, Aerts JMFG, Naim HY, Zimmer KP. Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher's disease. Int J Biochem Cell Biol. 2005;37(11):2310–20.
Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14(16):2387–98.
Michelakakis H, Xiromerisiou G, Dardiotis E, Bozi M, Vassilatis D, Kountra PM, et al. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson's disease. Mov Disord. 2012;27(3):400–5.
Alcalay RN, Levy OA, Wolf P, Oliva P, Zhang XK, Waters CH, et al. SCARB2 variants and glucocerebrosidase activity in Parkinson's disease. NPJ Parkinsons Dis. 2016;2:16004.
Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7(6):e1002141.
Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014;23(23):6139–46.
Salvioli R, Tatti M, Scarpa S, Moavero SM, Ciaffoni F, Felicetti F, et al. The N370S (Asn370-->Ser) mutation affects the capacity of glucosylceramidase to interact with anionic phospholipid-containing membranes and saposin C. Biochem J. 2005;390(Pt 1):95–103.
Atrian S, López-Viñas E, Gómez-Puertas P, Chabás A, Vilageliu L, Grinberg D. An evolutionary and structure-based docking model for glucocerebrosidase-saposin C and glucocerebrosidase-substrate interactions - relevance for Gaucher disease. Proteins. 2008;70(3):882–91.
Lieberman RL, Wustman BA, Huertas P, Powe AC, Pine CW, Khanna R, et al. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3(2):101–7.
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52.
Yang J, Hertz E, Zhang X, Leinartaité L, Lundius EG, Li J, et al. Overexpression of α-synuclein simultaneously increases glutamate NMDA receptor phosphorylation and reduces glucocerebrosidase activity. Neurosci Lett. 2016;611:51–8.
Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, et al. Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson's disease. Brain. 2014;137(Pt 3):834–48.
Chiasserini D, Paciotti S, Eusebi P, Persichetti E, Tasegian A, Kurzawa-Akanbi M, et al. Selective loss of glucocerebrosidase activity in sporadic Parkinson's disease and dementia with Lewy bodies. Mol Neurodegener. 2015;10:15.
Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72(3):455–63.
Parnetti L, Paciotti S, Eusebi P, Dardis A, Zampieri S, Chiasserini D, et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in parkinson's disease patients. Mov Disord. 2017;32(10):1423–31.
Zunke F, et al. Reversible Conformational Conversion of a-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron. 97(1):92–107. e10
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–8.
Shimura H, Hattori N, Kubo SI, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25(3):302–5.
Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid Redox Signal. 2007;9(5):553–61.
Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19(19):3771–81.
Sunwoo M-K, Kim SM, Lee S, Lee PH. Parkinsonism associated with glucocerebrosidase mutation. J Clin Neurol. 2011;7(2):99–101.
Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275(23):5767–73.
Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology. 2013;80(17):1606–10.
Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson's disease. Brain. 2017;140(12):3191–203.
Gan-Or Z, Orr-Urtreger A, Alcalay RN, Bressman S, Giladi N, Rouleau GA. The emerging role of SMPD1 mutations in Parkinson's disease: implications for future studies. Parkinsonism Relat Disord. 2015;21(10):1294–5.
Grassmé H, Riethmüller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46(3):161–70.
Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA, et al. Acid β-glucosidase 1 counteracts p38δ-dependent induction of Interleukin-6 possible role for ceramide as an anti-inflammatory lipid. J Biol Chem. 2009;284(19):12979–88.
Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18(19):5252–63.
Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–87.
•• Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson's disease: the mutation matters. Ann Neurol. 2016;80(5):662–73. The authors compared PD patients with and without GBA mutations longitudinally, and showed that mutation carriers had a greater risk for dementia (hazard ratio = 3.16) and death (hazard ratio = 1.85) than noncarriers, and that carriers of severe mutations had greater risk for dementia than carriers of mild mutations but similar mortality risk.
Thaler A, Gurevich T, Bar Shira A, Gana Weisz M, Ash E, Shiner T, et al. A "dose" effect of mutations in the GBA gene on Parkinson's disease phenotype. Parkinsonism Relat Disord. 2017;36:47–51.
• Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, et al. GBA-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407–11. This study examines a cohort of PD patients with and without GBA mutations over 3 years, finding that GBA PD patients had earlier age of onset, faster motor progression, and reduced survival rates compared to non-carriers.
Jesus S, et al. GBA variants influence motor and non-motor features of Parkinson's disease. PLoS One. 2016;11(12):e0167749.
Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70(7):852–8.
Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77(3):276–80.
Li Y, Sekine T, Funayama M, Li L, Yoshino H, Nishioka K, et al. Clinicogenetic study of GBA mutations in patients with familial Parkinson's disease. Neurobiol Aging. 2014;35(4):935.e3–8.
• Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, et al. Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's. Ann Neurol. 2016;80(5):674–85. A total of 2,304 PD patients from 7 cohorts were followed for up to 12.8 years, showing that GBA mutation carriers were more likely to develop cognitive impairment, as measured by the mini-mental state exam, than non-carriers. Cognitive decline was faster in carriers of severe mutations in GBA compared to mild ones.
Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, van Deerlin VM, et al. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord. 2016;31(1):95–102.
Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with rapid eye movement sleep behavior disorder. Ann Clin Transl Neurol. 2015;2(9):941–5.
Parkkinen L, Neumann J, O'Sullivan SS, Holton JL, Revesz T, Hardy J, et al. Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson's disease. Mol Genet Metab. 2011;103(4):410–2.
Angeli A, Mencacci NE, Duran R, Aviles-Olmos I, Kefalopoulou Z, Candelario J, et al. Genotype and phenotype in Parkinson's disease: lessons in heterogeneity from deep brain stimulation. Mov Disord. 2013;28(10):1370–5.
Pal GD, Hall D, Ouyang B, Phelps J, Alcalay R, Pauciulo MW, et al. Genetic and clinical predictors of deep brain stimulation in young-onset Parkinson's disease. Mov Disord Clin Pract. 2016;3(5):465–71.
Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, et al. Comparison of parkinson risk in ashkenazi jewish patients with gaucher disease and gba heterozygotes. JAMA Neurol. 2014;71(6):752–7.
Arkadir D, Dinur T, Mullin S, Mehta A, Baris HN, Alcalay RN, et al. Trio approach reveals higher risk of PD in carriers of severe vs. mild GBA mutations. Blood Cells Mol Dis. 2018;68:115–6.
Hogl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol. 2018;14(1):40–55.
Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23(12):1665–72.
Anang JB, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253–60.
Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434–40.
Fereshtehnejad SM, Romenets SR, Anang JBM, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015;72(8):863–73.
Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79(10):1117–21.
Kumar KR, Ramirez A, Göbel A, Kresojević N, Svetel M, Lohmann K, et al. Glucocerebrosidase mutations in a Serbian Parkinson's disease population. Eur J Neurol. 2013;20(2):402–5.
Gamez-Valero A, et al. Glucocerebrosidase gene variants are accumulated in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2018;50:94–8.
Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C, et al. Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40(8)
Noreau A, Rivière JB, Diab S, Dion PA, Panisset M, Soland V, et al. Glucocerebrosidase mutations in a French-Canadian Parkinson's disease cohort. Can J Neurol Sci. 2011;38(5):772–3.
Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AHV. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72(2):201–8.
Nishioka K, Ross OA, Vilariño-Güell C, Cobb SA, Kachergus JM, Mann DMA, et al. Glucocerebrosidase mutations in diffuse Lewy body disease. Parkinsonism Relat Disord. 2011;17(1):55–7.
Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E, et al. REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov Disord. 2015;30(10):1413–7.
Shiner T, Mirelman A, Gana Weisz M, Bar-Shira A, Ash E, Cialic R, et al. High frequency of GBA gene mutations in dementia with Lewy bodies among Ashkenazi Jews. JAMA Neurol. 2016;73(12):1448–53.
Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–35.
Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012;79(19):1944–50.
Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55–65.
Clark LN, Chan R, Cheng R, Liu X, Park N, Parmalee N, et al. Gene-wise association of variants in four lysosomal storage disorder genes in neuropathologically confirmed Lewy body disease. PLoS One. 2015;10(5):e0125204.
Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VMY, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–10.
Segarane B, Li A, Paudel R, Scholz S, Neumann J, Lees A, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72(13):1185–6.
Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H. Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and corticobasal degeneration in polish patients. J Neurol. 2010;257(3):459–60.
Sun QY, Guo JF, Han WW, Zuo X, Wang L, Yao LY, et al. Genetic association study of glucocerebrosidase gene L444P mutation in essential tremor and multiple system atrophy in mainland China. J Clin Neurosci. 2013;20(2):217–9.
Srulijes K, Hauser AK, Guella I, Asselta R, Brockmann K, Schulte C, et al. No association of GBA mutations and multiple system atrophy. Eur J Neurol. 2013;20(4):e61–2.
Mitsui J, Matsukawa T, Sasaki H, Yabe I, Matsushima M, Dürr A, et al. Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol. 2015;2(4):417–26.
Sklerov M, Kang UJ, Liong C, Clark L, Marder K, Pauciulo M, et al. Frequency of GBA variants in autopsy-proven multiple system atrophy. Mov Disord Clin Pract. 2017;4(4):574–81.
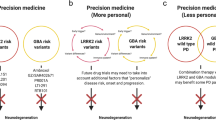
Giladi N, et al. A personalized approach to Parkinson’s disease patients based on founder mutation analysis. Front Neurol. 2016;7:71.
Sokol LL, Young MJ, Jankovic J. Counseling at-risk Parkinson’s disease cohorts: integrating emerging evidence. Curr Genet Med Rep. 2017;5(2):100–7.
Sakanaka K, Waters CH, Levy OA, Louis ED, Chung WK, Marder KS, et al. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns. 2014;23(1):114–20.
Falcone DC, Wood EMC, Xie SX, Siderowf A, van Deerlin VM. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. J Genet Couns. 2011;20(4):384–95.
Tan EK, Lee J, Hunter C, Shinawi L, Fook-Chong S, Jankovic J. Comparing knowledge and attitudes towards genetic testing in Parkinson's disease in an American and Asian population. J Neurol Sci. 2007;252(2):113–20.
Migdalska-Richards A, Daly L, Bezard E, Schapira AHV. Ambroxol effects in glucocerebrosidase and alpha-synuclein transgenic mice. Ann Neurol. 2016;80(5):766–75.
Maegawa GH, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284(35):23502–16.
Cook L, Schulze J. Connecting Gaucher and Parkinson disease: considerations for clinical and research genetic counseling settings. J Genet Couns. 2017;26(6):1165–72.
Mulhern M, Bier L, Alcalay RN, Balwani M. Patients' opinions on genetic counseling on the increased risk of Parkinson disease among Gaucher disease carriers. J Genet Couns. 2018;27(3):675–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ziv Gan-Or is supported by research grants from the Michael J. Fox Foundation, the Canadian Consortium on Neurodegeneration in Aging (CCNA), the Canadian Glycomics Network (GlycoNet), and the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) program. Dr. Gan-Or is consulting for Sanofi and for Lysosomal Therapeutics Inc. (LTI).
Christopher Liong declares no potential conflicts of interest.
Roy N. Alcalay is supported by the Parkinson’s Disease Foundation, the Michael J. Fox Foundation, and the National Institutes of Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Gan-Or, Z., Liong, C. & Alcalay, R.N. GBA-Associated Parkinson’s Disease and Other Synucleinopathies. Curr Neurol Neurosci Rep 18, 44 (2018). https://doi.org/10.1007/s11910-018-0860-4
Published:
DOI: https://doi.org/10.1007/s11910-018-0860-4