Abstract
Purpose
Intraoperative imaging aims at identifying residual tumor during surgery. Positron Surface Imaging (PSI) is one of the solutions to help surgeons in a better detection of resection margins of brain tumor, leading to an improved patient outcome. This system relies on a tracked freehand beta probe, using \(^{18}\)F-based radiotracer. Some acquisition models have been proposed in the literature in order to enhance image quality, but no comparative validation study has been performed for PSI.
Methods
In this study, we investigated the performance of different acquisition models by considering validation criteria and normalized metrics. We proposed a reference-based validation framework to perform the comparative study between acquisition models and a basic method. We estimated the performance of several acquisition models in light of four validation criteria: efficiency, computational speed, spatial accuracy and tumor contrast.
Results
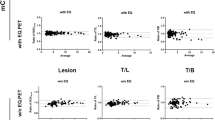
Selected acquisition models outperformed the basic method, albeit with the real-time aspect compromised. One acquisition model yielded the best performance among all according to the validation criteria: efficiency (1-Spe: 0.1, Se: 0.94), spatial accuracy (max Dice: 0.77) and tumor contrast (max T/B: 5.2). We also found out that above a minimum threshold value of the sampling rate, the reconstruction quality does not vary significantly.
Conclusion
Our method allowed the comparison of different acquisition models and highlighted one of them according to our validation criteria. This novel approach can be extended to 3D datasets, for validation of future acquisition models dedicated to intraoperative guidance of brain surgery.
















Similar content being viewed by others
Notes
A suxel is defined as the smallest element of the SOI.
References
Behbahaninia M, Martirosyan NL, Georges J, Udovich JA, Kalani MYS, Feuerstein BG, Nakaji P, Spetzler RF, Preul MC (2013) Intraoperative fluorescent imaging of intracranial tumors: a review. Clin Neurol Neurosurg 115(5):517–528
Daghighian F, Mazziotta JC, Hoffman EJ, Shenderov P, Eshaghian B, Siegel S, Phelps ME (1994) Intraoperative beta probe: a device for detecting tissue labeled with positron or electron emitting isotopes during surgery. Med Phys 21(1):153–157
Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD (2015) How slow is too slow? correlation of operative time to complications: an analysis from the tennessee surgical quality collaborative. J Am Coll Surg 220(4):550–558
Gerganov VM, Samii A, Giordano M, Samii M, Fahlbusch R (2011) Two-dimensional high-end ultrasound imaging compared to intraoperative mri during resection of low-grade gliomas. J Clin Neurosci 18(5):669–673
Grosu A, Weber W (2010) Pet for radiation treatment planning of brain tumours. Radiother Oncol 96(3):325
Hartl A, Shakir DI, Lasser T, Ziegler SI, Navab N (2015) Detection models for freehand spect reconstruction. Phys Med Biol 60(3):1031
Hata N, Muragaki Y, Inomata T, Maruyama T, Iseki H, Hori T, Dohi T (2005) Intraoperative tumor segmentation and volume measurement in mri-guided glioma surgery for tumor resection rate control. Acad Radiol 12(1):116–122
Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, Skrap M (2012) Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. a single-institution experience in 190 patients: clinical article. J Neurosurg 117(6):1039–1052
Jannin P, Korb W (2008) Assessment of image-guided interventions. Image-guided interventions. Springer, Berlin, pp 531–549
Jannin P, Grova C, Maurer CR Jr (2006) Model for defining and reporting reference-based validation protocols in medical image processing. Int J Comput Assist Radiol Surg 1(2):63–73
Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, Hv Santbrink (2011) Intraoperative mri-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12(11):1062–1070
Maier-Hein L, Groch A, Bartoli A, Bodenstedt S, Boissonnat G, Chang PL, Clancy N, Elson D, Haase S, Heim E, Hornegger J, Jannin P, Kenngott H, Kilgus T, Müller-Stich B, Oladokun D, Röhl S, dos Santos T, Schlemmer HP, Seitel A, Speidel S, Wagner M, Stoyanov D (2014) Comparative validation of single-shot optical techniques for laparoscopic 3-d surface reconstruction. Med Imag IEEE Trans 33(10):1913–1930
Matthies P, Gardiazabal J, Okur A, Vogel J, Lasser T, Navab N (2014) Mini gamma cameras for intra-operative nuclear tomographic reconstruction. Med Image Anal 18(8):1329–1336
McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Quinoñes-Hinojosa A (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63(4):700–708
Monge F, Shakir DI, Navab N, Jannin P (2016) Partition-based acquisition model for speed up navigated beta-probe surface imaging. In: SPIE medical imaging 2016: image-guided procedures, robotic interventions, and modeling, vol 9786. International Society for Optics and Photonics, San Diego, p 97862P
Navab N, Traub J, Wendler T, Buck A, Ziegler S (2008) Navigated nuclear probes for intra-operative functional imaging. In: 5th IEEE international symposium on, biomedical imaging: From Nano to Macro, 2008. ISBI 2008. pp 1395–1398
Oezguer C, Bieniarz J, Lasser T, Ziegler S, Navab N, Wendler T (2009) Phenomenological models for intraoperative positron emission surface imaging using handheld probes. In: World Congress on Medical Physics and Biomedical Engineering, Sept 7–12, 2009. Munich, Germany, Springer, pp 213–216
O’Neill K, Lyons SK, Gallagher WM, Curran KM, Byrne AT (2010) Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol 220(3):317–327
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H, Tellmann L, Jansen P, Reifenberger G, Hamacher K, Coenen HH, Langen KJ (2009) Comparison of 18f-fet and 18f-fdg pet in brain tumors. Nucl Med Biol 36(7):779–787
Roessler K, Becherer A, Donat M, Cejna M, Zachenhofer I (2012) Intraoperative tissue fluorescence using 5-aminolevolinic acid (5-ala) is more sensitive than contrast mri or amino acid positron emission tomography (18f-fet pet) in glioblastoma surgery. Neurol Res 34(3):314–317
Selbekk T, Brekken R, Indergaard M, Solheim O, Unsgard G (2012) Comparison of contrast in brightness mode and strain ultrasonography of glial brain tumours. BMC Med Imag 12(1):11. doi:10.1186/1471-2342-12-11
Shakir DI, Navab N, Ziegler SI (2010) Acquisition model for iterative reconstruction of navigated beta-probe surface images. In: Proceedings of IEEE nuclear science symposium and medical imaging conference (IEEE NSS-MIC), institute of electrical and electronics engineers, Knoxville, TN, USA
Shakir DI, Hartl A, Navab N, Ziegler SI (2011) Evaluation of an ad hoc model of detection physics for navigated beta-probe surface imaging. In: SPIE medical imaging, vol 7964. International Society for Optics and Photonics, Orlando, p 796405
Shakir DI, Hartl A, Schneider FR, Pulko J, Ziegler SI, Navab N, Lasser T (2012) Two new ad-hoc models of detection physics and their evaluation for navigated beta probe surface imaging. In: SPIE medical imaging, international society for optics and photonics, pp 83,162G
Shepp LA, Vardi Y (1982) Maximum likelihood reconstruction for emission tomography. Med Imag IEEE Trans 1(2):113–122
Šteňo A, Karlík M, Mendel P, Čík M, Šteňo J (2012) Navigated three-dimensional intraoperative ultrasound-guided awake resection of low-grade glioma partially infiltrating optic radiation. Acta Neurochirur 154(7):1255–1262
Tiffen JC, Bailey CG, Ng C, Rasko JE, Holst J (2010) Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol Cancer 9(1):299
Wendler T, Hartl A, Lasser T, Traub J, Daghighian F, Ziegler SI, Navab N (2007) Towards intra-operative 3d nuclear imaging: reconstruction of 3d radioactive distributions using tracked gamma probes. Medical image computing and computer-assisted intervention–MICCAI. Springer, Berlin, pp 909–917
Acknowledgments
The authors would like to thanks CAMPUS FRANCE (PROCOPE Program), Rennes Métropole and Le Ministère des Affaires étrangères et du Développement international for French-Bavaria student exchanges and also the French Cancer League for material support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This articles does not contain patient data.
Rights and permissions
About this article
Cite this article
Monge, F., Shakir, D.I., Lejeune, F. et al. Acquisition models in intraoperative positron surface imaging. Int J CARS 12, 691–703 (2017). https://doi.org/10.1007/s11548-016-1487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-016-1487-z