Abstract
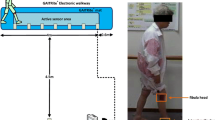
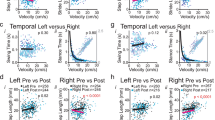
Gait disturbances are common in the rat model of Parkinson’s disease (PD) by administrating 6-hydroxydopamine. However, few studies have simultaneously assessed spatiotemporal gait indices and the kinematic information of PD rats during overground locomotion. This study utilized a simple, accurate, and reproducible method for quantifying the spatiotemporal and kinematic changes of gait patterns in hemiparkinsonian rats. A transparent walkway with a tilted mirror was set to capture underview footprints and lateral joint ankle images using a high-speed and high-resolution digital camera. The footprint images were semi-automatically processed with a threshold setting to identify the boundaries of soles and the critical points of each hindlimb for deriving the spatiotemporal and kinematic indices of gait. Following PD lesion, asymmetrical gait patterns including a significant decrease in the step/stride length and increases in the base of support and ankle joint angle were found. The increased footprint length, toe spread, and intermediary toe spread were found, indicating a compensatory gait pattern for impaired locomotor function. The temporal indices showed a significant decrease in the walking speed with increased durations of the stance/swing phase and double support time, which was more evident in the affected hindlimb. Furthermore, the ankle kinematic data showed that the joint angle decreased at the toe contact stage. We conclude that the proposed gait analysis method can be used to precisely detect locomotor function changes in PD rats, which is useful for objective assessments of investigating novel treatments for PD animal model.








Similar content being viewed by others
References
Almeida QJ, Lebold CA (2010) Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 81:513–518
Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG (2005) Gait dynamics in mouse models of Parkinson’s disease and Huntington’s disease. J Neuroeng Rehabil 2:20
Belluscio MA, Riquelme LA, Murer MG (2007) Striatal dysfunction increases basal ganglia output during motor cortex activation in parkinsonian rats. Eur J Neurosci 25:2791–2804
Bervar M (2000) Video analysis of standing—an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 102:109–116
Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT (2007) Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci 25:397–405
Blin O, Ferrandez AM, Serratrice G (1990) Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci 98:91–97
Bozkurt A, Scheffel J, Brook GA, Joosten EA, Suschek CV, O’Dey DM, Pallua N, Deumens R (2011) Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and CatWalk gait analysis. Behav Brain Res 219:55–62
Chang JY, Shi LH, Luo F, Woodward DJ (2006) Neural responses in multiple basal ganglia regions following unilateral dopamine depletion in behaving rats performing a treadmill locomotion task. Exp Brain Res 172:193–207
Chaniary KD, Baron MS, Rice AC, Wetzel PA, Ramakrishnan V, Shapiro SM (2009) Quantification of gait in dystonic Gunn rats. J Neurosci Methods 180:273–277
Chuang CS, Su HL, Cheng FC, Hsu SH, Chuang CF, Liu CS (2010) Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson’s disease. J Biomed Sci 17:9
Cousins MS, Salamone JD (1996) Involvement of ventrolateral striatal dopamine in movement initiation and execution: a microdialysis and behavioral investigation. Neuroscience 70:849–859
Couto PA, Filipe VM, Magalhaes LG, Pereira JE, Costa LM, Melo-Pinto P, Bulas-Cruz J, Mauricio AC, Geuna S, Varejao AS (2008) A comparison of two-dimensional and three-dimensional techniques for the determination of hindlimb kinematics during treadmill locomotion in rats following spinal cord injury. J Neurosci Methods 173:193–200
Dai H, Macarthur L, McAtee M, Hockenbury N, Das P, Bregman BS (2011) Delayed rehabilitation with task-specific therapies improves forelimb function after a cervical spinal cord injury. Restor Neurol Neurosci 29:91–103
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Dunnett SB, Iversen SD (1982) Spontaneous and drug-induced rotation following localized 6-hydroxydopamine and kainic acid-induced lesions of the neostriatum. Neuropharmacology 21:899–908
Fernagut PO, Diguet E, Labattu B, Tison F (2002) A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods 113:123–130
Fischer DA, Ferger B, Kuschinsky K (2002) Discrimination of morphine- and haloperidol-induced muscular rigidity and akinesia/catalepsy in simple tests in rats. Behav Brain Res 134:317–321
Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, Paleacu D, Korczyn AD (2001) Freezing of gait in patients with advanced Parkinson’s disease. J Neural Transm 108:53–61
Hefti F, Melamed E, Sahakian BJ, Wurtman RJ (1980) Circling behavior in rats with partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine, and DOPA. Pharmacol Biochem Behav 12:185–188
Hsieh TH, Chen JJ, Chen LH, Chiang PT, Lee HY (2011) Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav Brain Res 222:1–9
Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer BJ, Gerhardt GA (1993) Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 626:167–174
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162:1–10
Kim YK, Lim HH, Song YK, Lee HH, Lim S, Han SM, Kim CJ (2005) Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci Lett 384:133–138
Klein A, Wessolleck J, Papazoglou A, Metz GA, Nikkhah G (2009) Walking pattern analysis after unilateral 6-OHDA lesion and transplantation of foetal dopaminergic progenitor cells in rats. Behav Brain Res 199:317–325
Konitsiotis S, Tsironis C (2006) Levodopa-induced dyskinesia and rotational behavior in hemiparkinsonian rats: independent features or components of the same phenomenon? Behav Brain Res 170:337–341
Lin Y-C, Yang B-S, Lin Y-T, Yang Y-T (2011) Human recognition based on kinematics and kinetics of gait. J Med Bio Eng 31:255–263
Lindner MD, Plone MA, Francis JM, Emerich DF (1996) Validation of a rodent model of Parkinson’s disease: evidence of a therapeutic window for oral Sinemet. Brain Res Bull 39:367–372
Metz GA, Tse A, Ballermann M, Smith LK, Fouad K (2005) The unilateral 6-OHDA rat model of Parkinson’s disease revisited: an electromyographic and behavioural analysis. Eur J Neurosci 22:735–744
Miklyaeva EI, Martens DJ, Whishaw IQ (1995) Impairments and compensatory adjustments in spontaneous movement after unilateral dopamine depletion in rats. Brain Res 681:23–40
Muir GD, Whishaw IQ (1999) Ground reaction forces in locomoting hemi-parkinsonian rats: a definitive test for impairments and compensations. Exp Brain Res 126:307–314
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 5th edn. Elsevier Academic Press, Amsterdam
Pereira JE, Cabrita AM, Filipe VM, Bulas-Cruz J, Couto PA, Melo-Pinto P, Costa LM, Geuna S, Mauricio AC, Varejao AS (2006) A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav Brain Res 172:212–218
Reynolds JL, Urbanchek MS, Asato H, Kuzon WM Jr (1996) Deletion of individual muscles alters rat walking-track parameters. J Reconstr Microsurg 12:461–466
Rogers MW (1996) Disorders of posture, balance, and gait in Parkinson’s disease. Clin Geriatr Med 12:825–845
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787
Schneider JS, Peacock V (1998) Differential effects of GDNF treatment on rotational asymmetry, skilled forelimb use deficits and sensory neglect in unilateral 6-OHDA-lesioned rats. Restor Neurol Neurosci 13:205–212
Truong L, Allbutt H, Kassiou M, Henderson JM (2006) Developing a preclinical model of Parkinson’s disease: a study of behaviour in rats with graded 6-OHDA lesions. Behav Brain Res 169:1–9
Varejao AS, Cabrita AM, Patricio JA, Bulas-Cruz J, Gabriel RC, Melo-Pinto P, Couto PA, Meek MF (2001) Functional assessment of peripheral nerve recovery in the rat: gait kinematics. Microsurgery 21:383–388
Vlamings R, Visser-Vandewalle V, Koopmans G, Joosten EA, Kozan R, Kaplan S, Steinbusch HW, Temel Y (2007) High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience 148:815–823
Wada C, Sugimura Y, Ienaga T, Kimuro Y (2011) Basic research on the method of presenting distance information to the blind by means of gait measurement. J Med Bio Eng 31:283–287
Wang H, Spinner RJ, Sorenson EJ, Windebank AJ (2008) Measurement of forelimb function by digital video motion analysis in rat nerve transection models. J Peripher Nerv Syst 13:92–102
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, Kim SE, Lee HH, Kim YP, Kim CJ (2007) Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett 423:12–17
Yu P, Matloub HS, Sanger JR, Narini P (2001) Gait analysis in rats with peripheral nerve injury. Muscle Nerve 24:231–239
Acknowledgments
The authors acknowledge the National Science Council of Taiwan and National Health Research Institutes of Taiwan for financially supporting this work under grants NSC 96-2628-E-269-001-MY3, NHRI-EX 95-9524E1, and NHRI-EX98-9535EI.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.-Y. Lee and T.-H. Hsieh contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, HY., Hsieh, TH., Liang, JI. et al. Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med Biol Eng Comput 50, 937–946 (2012). https://doi.org/10.1007/s11517-012-0933-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-012-0933-5