Abstract
Purpose
Deficits in neuro-cognitive function are not uncommon for patients who have undergone surgical removal of brain tumors. Our goal is to evaluate the safety and efficacy of repetitive Transcranial Magnetic Stimulation (rTMS) as a non-invasive tool for the treatment of neuro-cognitive dysfunctions following craniotomy.
Methods
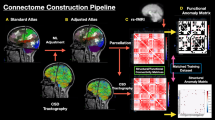
We present a retrospective review of individualized rTMS in twelve patients from Cingulum Health from December 2019 to July 2021 who presented with neuro-cognitive deficits following craniotomy. Multiple cortical targets were selected based on the patient’s neurological disorder, associated networks, and anomalies in the functional connectivity of the brain as determined by machine-learning. TMS treatment was performed for five consecutive days. EuroQol quality of life (EQ-5D), functional extremity scales, and neuropsychiatric questionnaires related to the patient’s deficit were assessed prior to, after, and during two-month follow-up of rTMS treatment.
Results
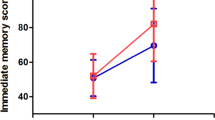
Nine patients had unilateral functional deficits in either upper, lower, or both limbs. One patient reported post-operative depression, another experienced short term memory difficulties, and a third reported hypobulia. All twelve patients reported significantly improved EQ5D after rTMS treatment and during follow-up. More than half of the patients with lower and upper functional deficits had a 9-point improvement during follow-up. In the patient who developed depression, an 88% reduction in depressive symptoms based on the Beck’s Depression Inventory (BDI) was observed during follow-up. No adverse events, such as seizures, occurred.
Conclusion
The personalized functional connectivity approach to rTMS treatment may be effective and safe for patients with post-craniotomy neuro-cognitive dysfunction.



Similar content being viewed by others
Data availability
Data is available upon request.
References
Chang SM et al (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 98(6):1175–1181
Litofsky NS et al (2004) Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery 54(2):358–366 (Discussion 366-367)
Starnoni D et al (2018) Returning to work after multimodal treatment in glioblastoma patients. Neurosurg Focus 44(6):E17
Gehring K et al (2009) Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol 27(22):3712–3722
Rapp SR et al (2015) Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol 33(15):1653–1659
De Witt Hamer PC et al (2021) Functional outcomes and health-related quality of life following glioma surgery. Neurosurgery 88(4):720–732
Peng Z et al (2018) Mechanism of repetitive transcranial magnetic stimulation for depression. Shanghai Arch Psychiatry 30(2):84–92
Hoyer EH, Celnik PA (2011) Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci 29(6):395–409
Cohen SL et al (2022) A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul 15(1):73–75
Poologaindran A et al (2022) Interventional neurorehabilitation for promoting functional recovery post-craniotomy: a proof-of-concept. Sci Rep 12(1):3039
Yeung JT et al (2021) Changes in the Brain Connectome Following Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation. Cureus 13(10):e19105-e
Jung J et al (2021) The immediate impact of transcranial magnetic stimulation on brain structure: Short-term neuroplasticity following one session of cTBS. Neuroimage 240:118375
Huang YZ et al (2005) Theta burst stimulation of the human motor cortex. Neuron 45(2):201–206
Sonmez AI et al (2019) Accelerated TMS for Depression: A systematic review and meta-analysis. Psychiatry Res 273:770–781
Chesworth BM et al (2014) Reliability and validity of two versions of the upper extremity functional index. Physiother Can 66(3):243–253
Binkley JM et al (1999) The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther 79(4):371–383
Gutner CA et al (2016) Emergence of transdiagnostic treatments for PTSD and posttraumatic distress. Curr Psychiatry Rep 18(10):95
Adachi Y et al (2012) Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex 22(7):1586–1592
Briggs RG et al (2021) The frontal aslant tract and supplementary motor area syndrome: moving towards a connectomic initiation axis. Cancers (Basel) 13(5):1116
Dadario NB et al (2021) Reducing the cognitive footprint of brain tumor surgery. Front Neurol 12:711646
Du J et al (2016) Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol 23(11):1666–1672
Tosun A et al (2017) Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil 24(5):361–367
Ille S et al (2021) Navigated repetitive transcranial magnetic stimulation improves the outcome of postsurgical paresis in glioma patients—a randomized, double-blinded trial. Brain Stimul 14(4):780–787
Kubis N (2016) Non-invasive brain stimulation to enhance post-stroke recovery. Front Neural Circuits 10:56
Chen W-H et al (2003) Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol 114(9):1628–1637
Jakola AS et al (2015) Perioperative quality of life in functionally dependent glioblastoma patients: a prospective study. Br J Neurosurg 29(6):843–849
Cole EJ et al (2020) Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry 177(8):716–726
Moreno-Ortega M et al (2020) Parcel-guided rTMS for depression. Transl Psychiatry 10(1):283
Singh A et al (2020) Default mode network alterations after intermittent theta burst stimulation in healthy subjects. Transl Psychiatry 10(1):75
Plewnia C et al (2021) Treatment of major depressive disorder with bilateral theta burst stimulation: study protocol for a randomized, double-blind, placebo-controlled multicenter trial (TBS-D). Eur Arch Psychiatry Clin Neurosci 271(7):1231–1243
McRae C et al (2004) Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry 61(4):412–420
Hoy KE et al (2019) A pilot investigation of repetitive transcranial magnetic stimulation for post-traumatic brain injury depression: safety, tolerability, and efficacy. J Neurotrauma 36(13):2092–2098
Li F et al (2019) Meta-analysis of placebo response in adult antidepressant trials. CNS Drugs 33(10):971–980
Prasser J et al (2015) Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry 16(1):57–65
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SJT: Formal Analysis, Writing—Original Draft; Writing—Review & Editing; Visualization. JH: Conceptualization; Methodology; Data Curation; Writing—Review & Editing; Visualization; Formal Analysis. OL: Conceptualization; Writing—Review & Editing. CT: Conceptualization; Writing—Review & Editing. MS: Conceptualization; Writing—Review & Editing. JY: Conceptualization; Methodology; Formal analysis; Writing—Review &Editing; Supervision. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
Charles Teo and Michael Sughrue are founders and employees of Omniscient Neurotechnology. Jonas Holle is an employee of Cingulum Health. Jacky Yeung and Olivia Lesslar are consultants of Cingulum Health but are not employees. Si Jie Tang does not report any conflicts of interest.
Ethical approval
This study was approved by the Human Research Ethics Committee of the South Eastern Sydney Local Health District (2022/ETH00139).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S.J., Holle, J., Lesslar, O. et al. Improving quality of life post-tumor craniotomy using personalized, parcel-guided TMS: safety and proof of concept. J Neurooncol 160, 413–422 (2022). https://doi.org/10.1007/s11060-022-04160-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04160-y