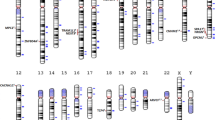
The etiology and pathogenesis of schizophrenia remain poorly understood, though it has been established that the contribution of heredity to the development of the disease is 80–85%. Over the past decade, significant progress has been made in the search for specific genetic variants associated with the development of schizophrenia. In this review, we discuss the results of recent large-scale studies seeking genetic associations with schizophrenia: genome-wide association studies (GWAS) and searches for rare variants (mutations or copy number variations, CNV), including studies using whole-exome sequencing. We synthesize data on currently known genes which are significantly associated with the development of schizophrenia and discuss their biological functions to identify the main molecular pathways involved in the pathophysiology of schizophrenia.
Similar content being viewed by others
References
T. Vos, S. S. Lim, C. Abbafati, et al., “Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019,” Lancet, 396, No. 10258, 1204–1222 (2020), https://doi.org/https://doi.org/10.1016/S0140-6736 (20)30925-9.
A. G. Cardno and I. I. Gottesman, “Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics,” Am. J. Med. Genet., 97, No. 1, 12–17 (2000).
R. Hilker, D. Helenius, B. Fagerlund, et al., “Heritability of schizophrenia and schizophrenia spectrum based on the Nationwide Danish Twin Register,” Biol. Psychiatry, 83, No. 6, 492–498 (2018), https://doi.org/https://doi.org/10.1016/j.biopsych.2017.08.017.
P. F. Sullivan, K. S. Kendler, and M. C. Neale, “Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies,” Arch. Gen. Psychiatry, 60, No. 12, 1187–1192 (2003), https://doi.org/https://doi.org/10.1001/archpsyc.60.12.1187.
V. Tam, N. Patel, M. Turcotte, et al., “Benefits and limitations of genome-wide association studies,” Nat. Rev. Genet., 20, No. 8, 467–484 (2019), https://doi.org/https://doi.org/10.1038/s41576-019-0127-1.
E. Uffelmann, Q. Q. Huang, N. S. Munung, et al., “Genome-wide association studies,” Nat. Rev. Methods Primers, 1, No. 1, 1–21 (2021), https://doi.org/https://doi.org/10.1038/s43586-021-00056-9.
W. S. Bush and J. H. Moore, “Chapter 11: Genome-wide association studies,” PLoS Comput. Biol., 8, No. 12, e1002822 (2012), https://doi.org/10.1371/journal.pcbi.1002822.
H. Stefansson, R. A. Ophoff, S. Steinberg, et al., “Common variants conferring risk of schizophrenia,” Nature, 460, No. 7256, 744–747 (2009), https://doi.org/https://doi.org/10.1038/nature08186.
The Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, “Genome-wide association study identifies five new schizophrenia loci,” Nat. Genet., 43, No. 10, 969–976 (2011), https://doi.org/10.1038/ng.940.
Schizophrenia Working Group of the Psychiatric Genomics Consortium, “Biological insights from 108 schizophrenia-associated genetic loci,” Nature, 511, No. 7510, 421–427 (2014), https://doi.org/https://doi.org/10.1038/nature13595.
A. F. Pardiñas, P. Holmans, A. J. Pocklington, et al., “Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection,” Nat. Genet., 50, No. 3, 381–389 (2018), https://doi.org/https://doi.org/10.1038/s41588-018-0059-2.
V. Trubetskoy, A. F. Pardiñas, T. Qi, et al., “Mapping genomic loci implicates genes and synaptic biology in schizophrenia,” Nature, 604, No. 7906, 502–508 (2022), https://doi.org/https://doi.org/10.1038/s41586-022-04434-5.
J. Pohlan, B. A. Leidel, and T. Lindner, “Neurogranin,” in: Biomarkers for Traumatic Brain Injury, A. H. B. Wu and W. F. Peacock (eds.), Academic Press (2020), Chpt. 5, pp. 211–219, https://doi.org/10.1016/B978-0-12-816346-7.00015-4.
J. H. Pak, F. L. Huang, J. Li, et al., “Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase, synaptic plasticity, and spatial learning: a study with knockout mice,” Proc. Natl. Acad. Sci. USA, 97, No. 21, 11232–11237 (2000), https://doi.org/https://doi.org/10.1073/pnas.210184697.
T. Miyakawa, E. Yared, J. H. Pak, et al., “Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components,” Hippocampus, 11, No. 6, 763–775 (2001), https://doi.org/https://doi.org/10.1002/hipo.1092.
T. Toulopoulou, M. Picchioni, F. Rijsdijk, et al., “Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples,” Arch. Gen. Psychiatry, 64, No. 12, 1348–1355 (2007), https://doi.org/https://doi.org/10.1001/archpsyc.64.12.1348.
K. Nakazawa and K. Sapkota, “The origin of NMDA receptor hypofunction in schizophrenia,” Pharmacol. Ther., 205, 107426 (2020), https://doi.org/https://doi.org/10.1016/j.pharmthera.2019.107426.
S. Mesman, R. Bakker, and Smidt, M. P., “Tcf4 is required for correct brain development during embryogenesis,” Mol. Cell. Neurosci., 106, 103502 (2020), https://doi.org/10.1016/j.mcn.2020.103502.
E. Mahmoudi and M. J. Cairns, “MiR-137: an important player in neural development and neoplastic transformation,” Mol. Psychiatry, 22, No. 1, 44–55 (2017), https://doi.org/https://doi.org/10.1038/mp.2016.150.
G. Sun, P. Ye, K. Murai, et al., “miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells,” Nat. Commun., 2, 529 (2011), https://doi.org/https://doi.org/10.1038/ncomms1532.
T. A. Lett, M. M. Chakravarty, M. M. Chakavarty, et al., “The genome- wide supported microRNA-137 variant predicts phenotypic heterogeneity within schizophrenia,” Mol. Psychiatry, 18, No. 4, 443–450 (2013), https://doi.org/https://doi.org/10.1038/mp.2013.17.
I. Guella, A. Sequeira, B. Rollins, et al., “Analysis of miR-137 expression and rs1625579 in dorsolateral prefrontal cortex,” J. Psychiatr. Res., 47, No. 9, 1215–1221 (2013), https://doi.org/https://doi.org/10.1016/j.jpsychires.2013.05.021.
L. Zhao, H. Li, R. Guo, et al., “miR-137, a new target for post-stroke depression?” Neural Regen. Res., 8, No. 26, 2441–2448 (2013), https://doi.org/https://doi.org/10.3969/j.issn.1673-5374.2013.26.005.
W. Hu, M. L. MacDonald, D. E. Elswick, and R. A. Sweet, “The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies,” Ann. N. Y. Acad. Sci., 1338, No. 1, 38–57 (2015), https://doi.org/https://doi.org/10.1111/nyas.12547.
A. A. Shmakova, K. A. Rubina, K. V. Anokhin, et al., “The role of plasminogen activator system in the pathogenesis of epilepsy,” Biochemistry (Mosc.), 84, No. 9, 979–991 (2019), https://doi.org/https://doi.org/10.1134/S0006297919090013.
B. Torrico, A. D. Shaw, R. Mosca, et al., “Truncating variant burden in high-functioning autism and pleiotropic effects of LRP1 across psychiatric phenotypes,” J. Psychiatr. Neurosci., 44, No. 5, 350–359 (2019), https://doi.org/https://doi.org/10.1503/jpn.180184.
T. A. Pollak, J. P. Rogers, R. G. Nagele, et al., “Antibodies in the diagnosis, prognosis, and prediction of psychotic disorders,” Schizophr. Bull., 45, No. 1, 233–246 (2019), https://doi.org/https://doi.org/10.1093/schbul/sby021.
O. M. Zakharenko, T. P. Kliushnik, I. A. Kozlova, et al., “Nerve growth factor auto-antibodies in the sera of mothers of schizophrenic children and children from high risk group,” Zh. Nevrol. Psikhiat., 99, No. 3, 44–46 (1999).
O. P. Shmakova, L. V. Androsova, A. A. Shmakova, et al., “Clinical immunological correlates of mental disorders not in exacerbation,” Psikhiatriya, 65, No. 1, 17–23 (2015).
M. Hussein and R. Magdy, “MicroRNAs in central nervous system disorders: current advances in pathogenesis and treatment,” Egypt, J. Neurol. Psychiatry Neurosurg., 57, No. 1, 36 (2021), https://doi.org/10.1186/s41983-021-00289-1.
A. A. Shmakova, K. D. Rysenkova, O. I. Ivashkina, et al., “Early induction of neurotrophin receptor and miRNA genes in mouse brain after pentilenetetrazole-induced neuronal activity,” Biochemistry (Mosc.), 86, No. 10, 1326–1341 (2021), https://doi.org/https://doi.org/10.1134/S0006297921100138.
E. V. Semina, K. D. Rysenkova, K. E. Troyanovskiy, et al., “Micro- RNAs in cancer: From gene expression regulation to the metastatic niche reprogramming,” Biochemistry (Mosc.), 86, No. 7, 785–799 (2021), https://doi.org/https://doi.org/10.1134/S0006297921070014.
D. D. Cao, L. Li, and Chan, W. Y., “MicroRNAs: Key regulators in the central nervous system and their implication in neurological diseases,” Int. J. Mol. Sci., 17, No. 6, 842 (2016), https://doi.org/10.3390/ijms17060842.
E. Semina, K. Rubina, V. Sysoeva, et al., “Urokinase and urokinase receptor participate in regulation of neuronal migration, axon growth and branching,” Eur. J. Cell Biol., 95, No. 9, 295–310 (2016), https://doi.org/https://doi.org/10.1016/j.ejcb.2016.05.003.
A. A. Shmakova, A. V. Balatskiy, M. A. Kulebyakina, et al., “Urokinase receptor uPAR overexpression in mouse brain stimulates the migration of neurons into the cortex during embryogenesis,” Russ. J. Dev. Biol., 52, No. 1, 53–63 (2021), https://doi.org/https://doi.org/10.1134/S1062360421010069.
A. A. Shmakova, K. A. Rubina, K. D. Rysenkova, et al., “Urokinase receptor and tissue plasminogen activator as immediate early genes in pentylenetetrazole-induced seizures in the mouse brain,” Eur. J. Neurosci., 51, No. 7, 1559–1572 (2020), https://doi.org/https://doi.org/10.1111/ejn.14584.
K. D. Rysenkova, P. S. Klimovich, A. A. Shmakova, et al., “Urokinase receptor deficiency results in EGFR-mediated failure to transmit signals for cell survival and neurite formation in mouse neuroblastoma cells,” Cell. Signal., 75, 109741–109741 (2020), https://doi.org/https://doi.org/10.1016/j.cellsig.2020.109741.
M. Lek, K. J. Karczewski, E. V. Minikel, et al., “Analysis of protein- coding genetic variation in 60,706 humans,” Nature, 536, No. 7616, 285–291 (2016), https://doi.org/https://doi.org/10.1038/nature19057.
A. L. Collins, Y. Kim, R. J. Bloom, et al., “Transcriptional targets of the schizophrenia risk gene MIR137,” Transl. Psychiatry, 4, No. 7, 404–404 (2014), https://doi.org/https://doi.org/10.1038/tp.2014.42.
K. A. Rubina, E. I. Surkova, E. V. Semina, et al., “T-Cadherin expression in melanoma cells stimulates stromal cell recruitment and invasion by regulating the expression of chemokines, integrins and adhesion molecules,” Cancers (Basel), 7, No. 3, 1349–1370 (2015).
K. A. Rubina, E. V. Semina, N. I. Kalinina, et al., “Revisiting the multiple roles of T cadherin in health and disease,” Eur. J. Cell Biol., 100, No. 7, 151183 (2021), https://doi.org/10.1016/j.ejcb.2021.151183.
J. Lasky-Su, B. M. Neale, B. Franke, et al., “Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations,” Am. J. Med. Genet. B. Neuropsychiatr. Genet., 1457B, No. 8, 1345–1354 (2008), https://doi.org/https://doi.org/10.1002/ajmg.b.30867.
A. Salatino-Oliveira, J. P. Genro, G. Polanczyk, et al., “Cadherin-13 gene is associated with hyperactive/impulsive symptoms in attention/deficit hyperactivity disorder,” Am. J. Med. Genet. B. Neuropsychiatr. Genet., 168, No. 3, 162–169 (2015), https://doi.org/https://doi.org/10.1002/ajmg.b.32293.
S. J. Sanders, A. G. Ercan-Sencicek, V. Hus, et al., “Multiple recurrent de novo copy number variations (CNVs), including duplications of the 7q11.23 Williams-Beuren syndrome region, are strongly associated with autism,” Neuron, 70, No. 5, 863–885 (2011), https://doi.org/https://doi.org/10.1016/j.neuron.2011.05.002.
S. J. Sanders, X. He, A. J. Willsey, et al., “Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci,” Neuron, 87, No. 6, 1215–1233 (2015), https://doi.org/https://doi.org/10.1016/j.neuron.2015.09.016.
M. Tantra, L. Guo, J. Kim, et al., “Conditional deletion of cadherin 13 perturbs Golgi cells and disrupts social and cognitive behaviors,” Genes Brain Behav., 17, No. 6, e12466 (2018), https://doi.org/10.1111/gbb.12466.
D. Holland, O. Frei, R. Desikan, et al., “Beyond SNP heritability: Polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model,” PLOS Genet., 16, No. 5, e1008612 (2020), https://doi.org/10.1371/journal.pgen.1008612.
T. Kanazawa, C. A. Bousman, C. Liu, and Everall, I. P., “Schizophrenia genetics in the genome-wide era: a review of Japanese studies,” NPJ Schizophr., 3, No. 1, 1–6, https://doi.org/10.1038/s41537-017-0028-2. (2017).
J. Gratten, N. R. Wray, M. C. Keller, and P. M. Visscher, “Largescale genomics unveils the genetic architecture of psychiatric disorders,” Nat. Neurosci., 17, No. 6, 782–790 (2014), https://doi.org/https://doi.org/10.1038/nn.3708.
T. Singh, T. Poterba, D. Curtis, et al., “Rare coding variants in ten genes confer substantial risk for schizophrenia,” Nature, 604, No. 7906, 509–516 (2022), https://doi.org/https://doi.org/10.1038/s41586-022-04556-w.
X. Zhou, J. M. Long, M. A. Geyer, et al., “Reduced expression of the Sp4 gene in mice causes deficits in sensorimotor gating and memory associated with hippocampal vacuolization,” Mol. Psychiatry, 10, No. 4, 393–406 (2005), https://doi.org/https://doi.org/10.1038/sj.mp.4001621.
X. Zhou, Y. Qyang, J. R. Kelsoe, et al., “Impaired postnatal development of hippocampal dentate gyrus in Sp4 null mutant mice,” Genes Brain Behav., 6, No. 3, 269–276 (2007), https://doi.org/https://doi.org/10.1111/j.1601-183X.2006.00256.x.
X. Wang, A. Pinto-Duarte, M. M. Behrens, et al., “Ketamine independently modulated power and phase-coupling of theta oscillations in Sp4 hypomorphic mice,” PLoS One, 13, No. 3, e0193446 (2018), https://doi.org/https://doi.org/10.1371/journal.pone.0193446.
Zhou, X., “Over-representation of potential SP4 target genes within schizophrenia-risk genes,” Mol. Psychiatry, 27, No. 2, 849–854 (2022), https://doi.org/https://doi.org/10.1038/s41380-021-01376-8.
T. Chano, H. Okabe, and Hulette, C. M., “RB1CC1 insufficiency causes neuronal atrophy through mTOR signaling alteration and involved in the pathology of Alzheimer’s diseases,” Brain Res., 1168, 97–105 (2007), https://doi.org/10.1016/j.brainres.2007.06.075.
C. C. Liang, C. Wang, X. Peng, et al., “Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration,” J. Biol. Chem., 285, No. 5, 3499–3509 (2010), https://doi.org/https://doi.org/10.1074/jbc.M109.072389.
C. Wang, C. C. Liang, Z. C. Bian, et al., “FIP200 is required for maintenance and differentiation of postnatal neural stem cells,” Nat. Neurosci., 16, No. 5, 532–542 (2013), https://doi.org/https://doi.org/10.1038/nn.3365.
J. Mukai, E. Cannavò, G. W. Crabtree, et al., “Recapitulation and reversal of schizophrenia-related phenotypes in Setd1a-deficient mice,” Neuron, 104, No. 3, 471–487.e12 (2019), https://doi.org/https://doi.org/10.1016/j.neuron.2019.09.014.
K. Nagahama, K. Sakoori, T. Watanabe, et al., “Setd1a insufficiency in mice attenuates excitatory synaptic function and recapitulates schizophrenia-related behavioral abnormalities,” Cell Rep., 32, No. 11, 108126 (2020), https://doi.org/10.1016/j.celrep.2020.108126.
R. Chen, Y. Liu, M. N. Djekidel, et al., “Cell type-specific mechanism of Setd1a heterozygosity in schizophrenia pathogenesis,” Sci. Adv., 8, No. 9, eabm1077 (2022), https://doi.org/10.1126/sciadv.abm1077.
K. D. Rysenkova, K. E. Troyanovskiy, P. S. Klimovich, et al., “Identification of a novel small RNA encoded in the mouse urokinase receptor uPAR gene (Plaur) and its molecular target Mef2d,” Front. Mol. Neurosci., 15 (2022), https://doi.org/10.3389/fnmol.2022.865858, https://www.frontiersin.org/articles/, acc. Aug. 7, 2022.
W. Ba, Y. Yan, M. R. F. Reijnders, et al., “TRIO loss of function is associated with mild intellectual disability and affects dendritic branching and synapse function,” Hum. Mol. Genet., 25, No. 5, 892–902 (2016), https://doi.org/https://doi.org/10.1093/hmg/ddv618.
S. M. Katrancha, Y. Wu, M. Zhu, et al., “Neurodevelopmental disease- associated de novo mutations and rare sequence variants affect TRIO GDP/GTP exchange factor activity,” Hum. Mol. Genet., 26, No. 23, 4728–4740 (2017), https://doi.org/https://doi.org/10.1093/hmg/ddx355.
S. Barbosa, S. Greville-Heygate, M. Bonnet, et al., “Opposite modulation of RAC1 by mutations in TRIO is associated with distinct, domain-specific neurodevelopmental disorders,” Am. J. Hum. Genet., 106, No. 3, 338–355 (2020), https://doi.org/https://doi.org/10.1016/j.ajhg.2020.01.018.
H. C. Archbold, K. L. Jackson, A. Arora, et al., “TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia,” Sci. Rep., 8, No. 1, 4606 (2018), https://doi.org/10.1038/s41598-018-22858-w.
O. Pös, J. Radvanszky, G. Buglyó, et al., “DNA copy number variation: Main characteristics, evolutionary significance, and pathological aspects,” Biomed. J., 44, No. 5, 548–559 (2021), https://doi.org/https://doi.org/10.1016/j.bj.2021.02.003.
A. K. Singh, M. F. Olsen, L. A. S. Lavik, et al., “Detecting copy number variation in next generation sequencing data from diagnostic gene panels,” BMC Med. Genomics, 14, No. 1, 214 (2021), https://doi.org/10.1186/s12920-021-01059-x.
C. F. Lin, A. C. Naj, and Wang, L. S., “Analyzing copy number variation using SNP array data: Protocols for calling CNV and association tests,” Curr. Protoc. Hum. Genet., 79, Unit-1.27 (2013), https://doi.org/10.1002/0471142905.hg0127s79.
C. R. Marshall, D. P. Howrigan, D. Merico, et al., “Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects,” Nat. Genet., 49, No. 1, 27–35 (2017), https://doi.org/https://doi.org/10.1038/ng.3725.
E. Michaelovsky, M. Carmel, A. Frisch, et al., “Risk gene-set and pathways in 22q11.2 deletion-related schizophrenia: a genealogical molecular approach,” Transl. Psychiatry, 9, No. 1, 1–9 (2019), https://doi.org/https://doi.org/10.1038/s41398-018-0354-9.
O. K. Vo, A. McNeill, and K. S. Vogt, “The psychosocial impact of 22q11 deletion syndrome on patients and families: A systematic review,” Am. J. Med. Genet. A, 176, No. 10, 2215–2225 (2018), https://doi.org/https://doi.org/10.1002/ajmg.a.38673.
M. Schneider, M. Debbané, A. S. Bassett, et al., “Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome,” Am. J. Psychiatry, 171, No. 6, 627–639 (2014), https://doi.org/https://doi.org/10.1176/appi.ajp.2013.13070864.
D. M. McDonald-McGinn, K. E. Sullivan, B. Marino, et al., ”22q11.2 deletion syndrome,” Nat. Rev. Dis. Primers, 1, No. 1, 1–19 (2015), https://doi.org/10.1038/nrdp.2015.71.
J. Mukai, M. Tamura, K. Fénelon, et al., “Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia,” Neuron, 86, No. 3, 680–695 (2015), https://doi.org/https://doi.org/10.1016/j.neuron.2015.04.003.
E. H. Barriga, D. N. Alasaadi, C. Mencarelli, et al., “RanBP1 plays an essential role in directed migration of neural crest cells during development,” publ. online May 7, 2022:2022.05.05.490747, https://doi.org/10.1101/2022.05.05.490747.
A. Sekar, A. R. Bialas, H. de Rivera, et al., “Schizophrenia risk from complex variation of complement component 4,” Nature, 530, No. 7589, 177–183 (2016), https://doi.org/https://doi.org/10.1038/nature16549.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 123, No. 2, Iss. 1, pp. 26–36, February, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shmakova, A.A., Semina, E.V., Neyfeld, E.A. et al. Analysis of the Relationship between Genetic Factors and the Risk of Schizophrenia. Neurosci Behav Physi 53, 1128–1138 (2023). https://doi.org/10.1007/s11055-023-01513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01513-6