Abstract
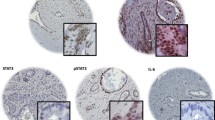
The transcription functions of oestrogen receptors (ER) are influenced by several coregulators such as PELP1 (proline, glutamate and leucine rich protein 1). The aim of the present study, which uses tissue microarrays and immunohistochemistry, is to explore the clinical and biological relevance of PELP1 protein expression in a large series of consecutive patients (1,162 patients) with invasive breast cancers with particular emphasis on its role in the ER-positive/luminal-like class of tumours. Our results showed that increased PELP1 expression is associated with tumours of larger size, higher histological grade, higher mitotic count, and with positive expression of basal cytokeratins (CK) (CK14; P = 0.018 and CK5/6; P = 0.029), P-cadherin (P = 0.002), p53 and MIB1 (P = 0.018). There was an inverse association between PELP1 expression and ER (P = 0.002), progesterone (PgR) (P = 0.004), androgen (AR) receptor (P < 0.001), and luminal CK (CK18; P = 0.027) expression. A significant association between PELP1 expression and shorter breast cancer specific survival (BCSS) (P = 0.002) and disease-free survival (DFI) (P = 0.006) was found. Multivariate Cox hazard analysis showed that PELP1 expression was an independent predictor of shorter BCSS (Hazard ratio (HR) = 1.349, P = 0.006) and shorter DFI (HR = 1.255, P = 0.011). In the ER-positive/luminal-like group (n = 768), PELP1 expression showed similar association with other clinicopathological variables and was an independent predictor of shorter DFI (HR = 1.256, P = 0.036). In conclusion, PELP1 protein expression is an independent prognostic predictor of shorter BCSS and DFI in breast cancer and its elevated expression is positively associated with markers of poor outcome. PELP1 appears to have a potential application in assessing the clinical outcome of patients with ER-positive breast cancer.


Similar content being viewed by others
References
Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A et al (2001) Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem 276:38272–38279
Brann DW, Zhang QG, Wang RM, Mahesh VB, Vadlamudi RK (2008) PELP1—a novel estrogen receptor-interacting protein. Mol Cell Endocrinol 290:2–7. doi:10.1016/j.mce.2008.04.019
Rajhans R, Nair HB, Nair SS, Cortez V, Ikuko K, Kirma NB et al (2008) Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: potential estrogen receptor autocrine signaling loop in breast cancer cells. Mol Endocrinol 22:649–664. doi:10.1210/me.2007-0350
Nagpal JK, Nair S, Chakravarty D, Rajhans R, Pothana S, Brann DW et al (2008) Growth factor regulation of estrogen receptor coregulator PELP1 functions via protein kinase a pathway. Mol Cancer Res 6:851–861. doi:10.1158/1541-7786.mcr-07-2030
Choi YB, Ko JK, Shin J (2004) The transcriptional corepressor, PELP1, recruits HDAC2 and masks histones using two separate domains. J Biol Chem 279:50930–50941. doi:10.1074/jbc.M406831200
Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK (2004) Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res 64:6416–6423. doi:10.1158/0008-5472.can-04-1786
Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK (2007) Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res 67:5505–5512. doi:10.1158/0008-5472.can-06-3647
Tzelepi V, Grivas P, Kefalopoulou Z, Kalofonos H, Varakis J, Sotiropoulou-Bonikou G (2009) Expression of estrogen receptor co-regulators NCoR and PELP1 in epithelial cells and myofibroblasts of colorectal carcinomas: cytoplasmic translocation of NCoR in epithelial cells correlates with worse prognosis. Virchows Arch 454(1):41–53. doi:10.1007/s00428-008-0708-4
Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JFR et al (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116:340–350. doi:10.1002/ijc.21004
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast-cancer. 1. The value of histological grade in breast-cancer—experience from a large study with long-term follow-up. Histopathology 19:403–410. doi:10.1111/j.1365-2559.1991.tb00229.x
Galea MH, Blamey RW, Elston CE, Ellis IO (1992) The Nottingham prognostic index in primary breast-cancer. Breast Cancer Res Treat 22:207–219. doi:10.1007/BF01840834
Habashy HO, Powe DG, Rakha EA, Ball G, Paish C, Gee J et al (2008) Forkhead-box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer 44:1541–1551. doi:10.1016/j.ejca.2008.04.020
Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR et al (2009) Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer ResTreat. doi:10.1007/s10549-009-0345-x
McCarty KS, Miller LS, Cox EB, Konrath J, McCarty KS (1985) Estrogen-receptor analyses—correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. doi:10.1158/1078-0432.ccr-04-0713
Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG et al (2009) Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res 15:2302–2310. doi:10.1158/1078-0432.ccr-08-2132
Naderi A, Teschendorff AE, Barbosa-Morais NL, Pinder SE, Green AR, Powe DG et al (2006) A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene 26:1507–1516. doi:10.1038/sj.onc.1209920
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752. doi:10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874. doi:10.1073/pnas.191367098
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423. doi:10.1073/pnas.0932692100
Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A et al (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100:10393–10398. doi:10.1073/pnas.1732912100
West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R et al (2001) Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci USA 98:11462–11467. doi:10.1073/pnas.201162998
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009. doi:10.1056/NEJMoa021967
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. doi:10.1038/415530a
Nair S, Vadlamudi RK (2007) Emerging significance of ER-coregulator PELP1/MNAR in cancer. Histol Histopathol 22:91–96
Stallcup MR, Kim JH, Teyssier C, Lee Y-H, Ma H, Chen D (2003) The roles of protein-protein interactions and protein methylation in transcriptional activation by nuclear receptors and their coactivators. J Steroid Biochem Mol Biol 85:139–145. doi:10.1016/S0960-0760(03)00222-X
Frietze S, Lupien M, Silver PA, Brown M (2008) CARM1 Regulates Estrogen-Stimulated Breast Cancer Growth through Up-regulation of E2F1. Cancer Res 68:301–306. doi:10.1158/0008-5472.can-07-1983
Schiff R, Massarweh S, Shou J, Bharwani L, Arpino G, Rimawi M et al (2005) Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol 56:10–20. doi:10.1007/s00280-005-0108-2
Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam H-S et al (2008) MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids 73:901–905. doi:10.1016/j.steroids.2007.12.028
Acknowledgments
We thank the Ministry of Higher Education (Egypt) for funding H. O. Habashy and E. A. Rakha and Breast Cancer Campaign for funding A Green.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habashy, H.O., Powe, D.G., Rakha, E.A. et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat 120, 603–612 (2010). https://doi.org/10.1007/s10549-009-0419-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0419-9