Abstract
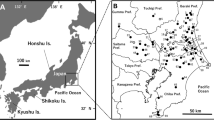
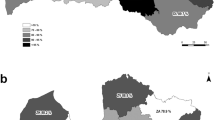
Prosthogonimiasis poses a threat to the reproductive system of poultry and wild birds, which are the definitive hosts of the parasite causing this disease. However, the parasite infection of the second intermediate host (dragonfly), the primary vector of this pathogen, is rarely reported. In this study, the prevalence of Prosthogonimus infection in dragonflies was investigated from June 2019 to October 2022 in Heilongjiang Province, northeast China. The species of metacercariae isolated from dragonfly were identified by morphological characteristics, molecular biology techniques, and animal infection experiments. The results showed that 11 species of dragonflies and one damselfly were identified and among six of the dragonflies infected by Prosthogonimus metacercariae, Sympetrum depressiusculum (28.53%) had the highest infection rate among all positive dragonflies, followed by Sympetrum vulgatum (27.86%) and Sympetrum frequens (20.99%), which are preferred hosts, and the total prevalence was 20.39% (2061/10,110) in Heilongjiang Province. Three species of Prosthogoniumus metacercariae were isolated, including Prosthogonimus cuneatus, Prosthogonimus pullucidus, and Prosthogonimus sp., among which P. cuneatus was the dominant species in dragonflies in Heilongjiang Province. This is the first report on the prevalence of Prosthogonimus in dragonflies in China, which provides baseline data for the control of prosthogonimiasis in Heilongjiang Province and a reference for the prevention of prosthogonimiasis in other areas of China.




Similar content being viewed by others
Data availability
Data supporting the conclusions of this article are included within the article and its additional files.
References
Acosta AA, Smit NJ, da Silva RJ (2020) Diversity of helminth parasites of eight siluriform fishes from the Aguapeí River, upper Paraná basin, São Paulo state. Brazil Int J Parasitol Parasites Wildl 11:120–128. https://doi.org/10.1016//j.ijppaw.2020.01.003
Arundel JH, Kingston JL, Kerr PJ (1980) Prosthogonimus pellucidus in domestic poultry. Australian Vet J 56:460–461. https://doi.org/10.1111/j.1751-0813.1980.tb02653.x
Asumang P, Akoto Delali J, Wiafe F, Kamil Z, Iddrisu Balali G, Afua Dela Gobe V, Nketiah Siaw W, Pinamang G (2019) Prevalence of gastrointestinal parasites in local and exotic breeds of chickens in Pankrono-Kumasi. Ghana J Parasitol Res 2019:1–7. https://doi.org/10.1155/2019/5746515
Beckstead RB, Anderson K, McDougald LR (2020) Oviduct fluke (Prosthogonimus macrorchis) found inside a chicken egg in North Carolina. Avian Dis 64:352–353. https://doi.org/10.1637/aviandiseases-D-20-00021
Bolek MG, Janovy J (2007) Evolutionary avenues for, and constraints on, the transmission of frog lung flukes (haematoloechus spp.) in dragonfly second intermediate hosts. J. Parasitol 93:593–607. https://doi.org/10.1645/GE-1011R.1
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. Revisited. J Parasitol. 83:575–583 (https://pubmed.ncbi.nlm.nih.gov/9267395)
Chen SY, Xiang H, Zhu X, Zhang H, Wang D, Liu HG, Wang JK, Yin T, Liu LQ, Kong MH, Zhang J, Ogura SI, Zhao XB (2018) Free dietary choice and free-range rearing improve the product quality, gait score, and microbial richness of chickens. Animals 8:84. https://doi.org/10.3390/ani8060084
Fecchio A, Chagas CRF, Bell JA, Kirchgatter K (2020) Evolutionary ecology, taxonomy, and systematics of avian malaria and related parasites. Acta Trop 204:105364. https://doi.org/10.1016/j.actatropica.2020.105364
Flores-Ferrer A, Marcou O, Waleckx E, Dumonteil E, Gourbière S (2018) Evolutionary ecology of chagas disease; what do we know and what do we need? Evol Appl 11:470–487. https://doi.org/10.1111/eva.12582
Gasser RB, Bott NJ, Chilton NB, Hunt P, Beveridge I (2008) Toward practical, DNA-based diagnostic methods for parasitic nematodes of livestock — Bionomic and biotechnological implications. Biotechnol Adv 26:325–334. https://doi.org/10.1016/j.biotechadv.2008.03.003
Guo XR, Li Y, Gao Y, Qiu YY, Jin ZH, Gao ZY, Zhang XG, An Q, Chang QC, Gao JF, Wang CR (2022) The complete mitochondrial genome of Prosthogonimus cuneatus and Prosthogonimus pellucidus (Trematoda: Prosthogonimidae), their features and phylogenetic relationships in the superfamily microphalloidea. Acta Trop 8:232. https://doi.org/10.1016/j.actatropica.2022.106469
Hao BC, Wang XH, Guo WZ, Guo ZT, Zhu YP, Hu YH, Liang JP (2011) Diagnosis and treatment of prosthogonimiasis infection in free-range laying hens. China Poultry 33:47–48 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99IB4TSWDnugoysoequixv2STBN30rAmCFCNAFNvNu7BWXRv1Wds1shNysYhGpo6WSoKmUX4jVxL-ZKVtotQ0XXXNOSEoJ3c7IXnFno66KJ4jwnf32_1q825RYze3YL2rjA=&uniplatform=NZKPT
Heneberg P, Jiljí S, Jiří B (2015) Integrative taxonomy of central European parasitic flatworms of the family Prosthogonimidae Lühe, 1909 (Trematoda: Plagiorchiida). Parasitol Int 64:264–273. https://doi.org/10.1016/j.parint.2015.02.003
Hong SJ, Woo HC, Lee SU, Huh S (1999) Infection status of dragonflies with Plagiorchis muris metacercariae in Korea. Korean J Parasitol 37:65. https://doi.org/10.3347/kjp.1999.37.2.65
Huang B, Shen J (2005) Classifier atlas of parasites for livestock and poultry in China. China Agricultural Science and Technology Press, China
Khudhair N, Yan C, Liu M, Yu H (2019) Effects of habitat types on macroinvertebrates assemblages structure: case study of sun island bund wetland. BioMed Res Int 1:13. https://doi.org/10.1155/2019/2650678
Lebedeva DI, Chrisanfova GG, Leshko EP, Guliaev AS, Yakovleva GA, Mendsaikhan B, Semyenova AS (2021) Morphological and molecular differentiation of Diplostomum spp. metacercariae from brain of minnows (Phoxinus phoxinus L.) in four populations of northern Europe and East Asia. Infect Genet Evol 92. https://doi.org/10.1016/j.meegid.2021.104911
Leok CS, Inoue I, Sato T, Haritani M, Tanimura N, Okada K (2002) Morphology of the oviduct fluke, Prosthogonimus ovatus, isolated from Indonesian native chickens and histopathological observation of the infected chickens. J Vet Med Sci 64:1129–1131. https://doi.org/10.1292/jvms.64.1129
Li Y, Zhang Y, Jin ZH, Wang LK, Gao ZY, Zhang XG, Wang CR, Shi TR (2021) Identification and evolutionary analysis of a species of Prosthogonimus spp. in Grus japonensis. Heilongjiang Ani Sci Vet Med 9:92–95 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99JfFwM1JkgTvHdhFwGlgPHt9pq4V8FvhSIuwOaxEG4hB4wf5OgfXqaDQfNZ7Uig6IPr61Bc1QVQhS5fhvNyxH0nWwt4ssfnyrIleSJrs72cPliBDOYjDFlj1w6xGNu7jxgMYNahzYs-BA==&uniplatform=NZKPT
Lin XY (2022) Diagnosis and experience of prosthogonimiasis in a free-range laying hen. Fujian J Ani Hus Vet Med 44:114–115 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99LJ8UFfIriS8daNdcwpmSzsmWiLPI-Um3_oynTscifEpW7RiXsSlcddm5UXRlLC_0l6z8EsANo1Mu8Mqqzh3GbN0nIfIQrrDZWsI2MAP78cVgHU7eDzgF98Iu-8Nua1hJlk5nbm-sRXfg==&uniplatform=NZKPT
Liu LW, Liao JS, Wu YB, Zhang YL (2020) Breeding range shift of the red-crowned crane (Grus japonensis) under climate change. PLoS One 15:e0229984. https://doi.org/10.1371/journal.pone.0229984
Luo JM, Ye YJ, Gao ZY, Wang WF, Hartup BK (2016) Lead in the red-crowned cranes (Grus japonensis) in Zhalong wetland, northeastern China: A report. Bull Environ Contam Toxicol 97:177–183. https://doi.org/10.1007/s00128-016-1853-0
Madsen H, Dung BT, The DT, Viet NK, Dalsgaard A, Van PT (2015) The role of rice fields, fish ponds and water canals for transmission of fish-borne zoonotic trematodes in aquaculture ponds in Nam Dinh Province. Vietnam Parasit Vectors 8:625. https://doi.org/10.1186/s13071-015-1237-z
McNew SM, Barrow LN, Williamson JL, Galen SC, Skeen HR, DuBay SG, Gaffney AM, Johnson AB, Bautista E, Ordoñez P, Schmitt CJ, Smiley A, Valqui T, Bates JM, Hackett SJ, Witt CC (2021) Contrasting drivers of diversity in hosts and parasites across the tropical Andes. Proc Natl Acad Sci U.S.A. 5:e2010714118. https://doi.org/10.1073/pnas.2010714118
Nguyen PTX, Van Hoang H, Dinh HTK, Dorny P, Losson B, Bui DT, Lempereur L (2021) Insights on foodborne zoonotic trematodes in freshwater snails in North and Central Vietnam. Parasitol Res 120:949–962. https://doi.org/10.1073/10.1007/s00436-020-07027-1
Nobles S, Jackson CR (2020) Effects of life stage, site, and species on the dragonfly gut microbiome. Microorganisms 8:183. https://doi.org/10.3390/microorganisms8020183
Novak CW, Goater TM (2013) Introduced bullfrogs and their parasites: Haematoloechus longiplexus (Trematoda) exploits diverse damselfly intermediate hosts on Vancouver island. J Parasitol 99:59–63. https://doi.org/10.3390/10.1645/GE-3145.1
Pilgrim EM, Roush SA, Krane DE (2002) Combining DNA sequences and morphology in systematics: testing the validity of the dragonfly species Cordulegaster bilineata. Heredity 89:184–190. https://doi.org/10.1038/sj.hdy.6800112
Prichard R (1997) Application of molecular biology in veterinary parasitology. Vet Parasitol 71:155–175. https://doi.org/10.1016/S0304-4017(97)00029-0
Prichard R, Tait A (2001) The role of molecular biology in veterinary parasitology. Vet Parasitol 98:169–194. https://doi.org/10.1016/s0304-4017(01)00429-0
Rząd I, Sitko J, Dzika E, Zalewski K, Śmietana P, Busse P (2020) Geographic and ecologic aspects of the community structure of trematodes of mallards (Anas platyrhynchos) in northern Poland and the Czech Republic. J Wildlife Dis 56:576. https://doi.org/10.7589/2019-02-041
Sadaf T, Javid A, Hussain A, Bukhari SM, Hussain SM, Ain Q, Ashraf S, Suleman S, Saleem M, Azam SM, Ahmad U, Ali W (2021) Studies on parasitic prevalence in pet birds from Punjab, Pakistan. Braz J Biol 83:e246229. https://doi.org/10.1590/1519-6984.246229
Schuster RK, Gajic B, Procter M, Wibbelt G, Ruibal BA, Qablan M (2022) Morphological and molecular characterization of Prosthogonimus falconis n. sp. (Trematoda; Prosthogonimidae), found in a peregrine falcon (Falco peregrinus) (Aves: Falconidae) in the United Arab Emirates. J Helminthol 96:e3. https://doi.org/10.1017/S0022149X2100078X
Sutanto AH (1974) Prosthogonimus sp. in an infant. Paediatr Indones 11:38–43 (https://pubmed.ncbi.nlm.nih.gov/4840984/)
Van CD, Doungchawee G, Suttiprapa S, Arimatsu Y, Kaewkes S, Sripa B (2017) Association between Opisthorchis viverrini and Leptospira spp. infection in endemic Northeast Thailand. Parasitol Int 66:503–509. https://doi.org/10.1016/j.parint.2016.10.006
Wang XY, Liu YW, Cai YL, Fan YX (2021) Methods of diagnosis and control of prosthogonimiasis in free-range chickens. Jilin Ani Hus Vet Med 42:51–52 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99KpEZBiXssylGrH4uEP2vCvdI09NK0U6bxZkhNvnoFyQiAc1soLvft_-qWU2xAnm62NROcu4eIIDcFVI2QIT1KBnvweRgcW7nvbV9_3B1wSYvh-7sNb3-xXHTC-PVdjALCisEKe2VN_pg==&uniplatform=NZKPT
Yang XL, Yang H, Yang X, Bai YH, Hou JY, Li JL (1997) The first found of Prosthogonimus cuneatus in Heilongjiang province. Vet Parasit Dis China 4:58–60 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99JMBsR7du9-pRKfi2Vuigm5pHP4F2t12yDLY-gfz0OJaVaQc6xG3vJ-zLUww4ooAcrN2q5P_KXvktAsDP73j70S8iOPRT8SHSffAho9eeDdhzOD0wWzOgDTl0fA3qamWgM=&uniplatform=NZKPT
Yin FB, Li Z, Mi RJ (1998) Diagnosis and treatment of chicken Prosthogonimus spp. China poultry 3:31 (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=f77bZMqd99Lhas1ypGshevtp6sx9rpdNZJ_GvWZtq4X3zF5e4LIEDg2ReJRmZp18I1FmvyTrywoUUJNwGLOmRL4oVHn-XpoySe-1Qkr7czG_QV_PXPFGQxUDbHytiObvSd0lq_WO23Q=&uniplatform=NZKPT
Zhang HM (2019) Dragonflies and damselflies of China. ChongQing University Press, China
Zhang Y, Chang QC, Zhang Y, Na L, Wang WT, Xu WW, Gao DZ, Liu ZX, Wang CR, Zhu XQ (2014) Prevalence of Clonorchis sinensis infection in freshwater fishes in northeastern China. Vet Parasitol 204:209–213. https://doi.org/10.1016/j.vetpar.2014.05.007
Funding
This work was supported by the National Natural Science Foundation of China (31972703, 32172886) and Natural Science Foundation of Heilongjiang Province (ZD2022C006).
Author information
Authors and Affiliations
Contributions
Ben Li: Data analysis, Writing – original draft, Software, Writing – review & editing. Zhuo Lan: Data analysis, Software. Xin-Ru Guo: Data analysis, Methodology. Ai-Hui Zhang: Samples collection. Wei Wei: Samples collection. Zhen-Hua Jin: Samples collection. Zhong-Yan Gao: Samples collection. Xian-Guang Zhang: Samples collection. Bai Li: Samples collection. Jun-Feng Gao: Samples collection. Chun-Ren Wang: Writing – review & editing.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Animal Ethics Committee of Heilongjiang Bayi Agricultural University (Approval No. HBAU2018-007).
Consent to participate
Done and confirmed.
Consent for publication
Done and confirmed.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, B., Lan, Z., Guo, XR. et al. Survey of the Prosthogonimus spp. metacercariae infection in the second intermediate host dragonfly in Heilongjiang Province, China. Parasitol Res 122, 2859–2870 (2023). https://doi.org/10.1007/s00436-023-07975-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07975-4