Abstract
We compare the microscopic anatomy of the mouthparts of representative species of Solifugae, Pseudoscorpiones and Parasitiformes (Acari). Specifically, we focus on the epistome, the labrum, the lateral lips (= endites of the pedipalpal coxae) and the musculature of the pharyngeal suction pump. We provide evidence that the labrum is reduced in Solifugae, but present and functional in Pseudoscorpiones and Acari. The epistome constitutes the entire dorsal face of the rostrosoma in Solifugae, but is internalized into the prosoma in Pseudoscorpiones. In Acari, the epistome shows an ancestral morphology, probably close to the ground pattern of chelicerates. The lateral lips of Solifugae contribute to the ventral face of the rostrosoma and the two lips of the mouth opening. In Solifugae, the ventral rostrosoma also includes a sclerite that might derive from a tritosternum. In Pseudoscorpiones, the lateral lips remain independent of the rostrosoma, they interlock ventral to the rostrosoma forming a perioral space. Here, the rostrosoma has an unpaired ventral lip of unresolved morphological origin, which is, however, clearly distinct from the lateral lips of Solifugae. The pharyngeal suction pump differs in all three clades in attachment, number of muscles and origin of muscles. We interpret the data as evidence for independent, parallel evolution of elements of the ground pattern of the (eu)chelicerate mouth parts. Based on the morphological elements of a common euchelicerate ground plan, the rostrosoma evolved independently in the three clades. We reject earlier hypotheses that consider the rostrosoma a character to support a phylogenetic relationship of the three clades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All euchelicerates feed on liquified prey (extraintestinal digestion). In many taxa, endites of the pedipalpal coxae form a “lower lip” to allow for intake of liquid prey. However, in some taxa (e.g., Palpigradi, Solifugae, Pseudoscorpiones, Parasitiformes) a rostrosoma evolved, i.e., an elongate “snout”, that is inserted into the prey to suck in liquified food. A detailed microscopic anatomical description of the rostrosoma exists only for the Palpigradi (Franz-Guess and Starck 2020). From a comparative morphological point of view, the rostrosoma is an interesting structure because it allows to test ideas about homology and parallel evolution. In external examination, the rostrosoma appears as a “snout” that is inserted into liquified food, but it provides little information about its evolutionary history and from which morphological elements of the prosoma it actually derives. Therefore, we studied the microscopic anatomy of the rostrosoma of Solifugae, Pseudoscorpiones and a representative of the Parasitiformes, to analyze which structures built the rostrosoma in these three groups. The results can then be compared with the already known microscopic anatomy of the rostrosoma of Palpigradi and interpreted in an evolutionary context. We consider this an exercise in comparative microscopic anatomy that helps elucidating the evolutionary morphology of a functionally similar structure.
A detailed comparative morphological study of the rostrosoma is also of interest because the generally accepted phylogenetic relationship of Solifugae and Pseudoscorpiones as sister taxa suggests that at least their rostrosoma is an autapomorphic character (Shultz 1990, 2007). Originally, Börner (1904) suggested the taxonomic assignment of Solifugae and Pseudoscorpiones as Haplocnemata, based on the lack of a patella in the legs of both taxa. van der Hammen (1977, 1986a, b, 1989) referred to Pseudoscorpiones and Solifugae as “Apatellata” (including a fossil opilionid, Krustarachne). Weygoldt and Paulus (1979), Weygoldt (1998), and Ax (2003) used morphological character analyses and placed the taxon Haplocnemata in a strict phylogenetic system. Shultz (1990, 2007) added the rostrum (= rostrosoma), which he considered a structure that develops from either the labrum or the epistome merging with a midventral sternapophysis (tritosternum) and the pedipalpal coxae. Giribet et al. (2002) reported consistent support for a clade Haplocnemata in a combined analysis using morphological and molecular data.
The morphology based phylogenetic relationship between both taxa has been supported by several molecular studies (review in Giribet 2018). However, other molecular studies (Sharma et al. 2014; Lozano-Fernandez et al. 2019; Howard et al. 2020) did not support the Haplocnemata hypothesis. Giribet (2018) placed Solifugae and Pseudoscorpiones on an unresolved polytomy at the basis of Arachnida.
In a comparative morphological study of the rostrosoma, Dunlop (2000) suggested the occurrence of a rostrosoma in Acari, i.e., an elongate anterior body region that is inserted into prey to ingest liquefied prey as shared derived characters of an unnamed taxon comprising Acari and Haplocnemata. Dunlop (2000) suggested that the epistomo-labral plate, which is the fused epistome and labrum, forms the dorsal element of the rostrosoma. The lateral lips are considered autapomorphic characters of Haplocnemata and have been homologized with coxal lobes (= coxal endites; Dunlop 2000) of the pedipalps in Opilioacarus, which are then considered homologous to the basal part of the gnathosoma and the hypostome of Acari (Dunlop 2000).
In this article, we compare the microscopic anatomy of the rostrosoma (mouthparts) of Solifugae, Pseudoscorpiones and Parasitiformes (as representative of the “Acari”) and analyze it for their morphological building blocks. Of course, we are restricted to exemplar species and some of the inferred phylogenetic conclusions will be based on the assumption that our descriptions represent a clade specific pattern. We account for taxon-specific morphological variability and discuss comparative data from the literature.
We test the following hypotheses: (1) The rostrosoma of Solifugae, Pseudoscorpiones and Acari are homologous, i.e., the dorsal part of the rostrosoma develops as a merged epistome and labrum (epistomo-labral plate) the ventral part (lateral lips) from endites of the pedipalpal coxae (Dunlop 2000). (2) The rostrosoma evolved independently in all three groups (parallel evolution), but derived from a common ground pattern (epistome, labrum and palpal endites). Parallel evolution of homologous elements [i.e., parallelism in Hall (2007)]. (3) The rostrosoma evolved convergent in all three groups and can be derived from unrelated structures [i.e., homoplasy; Wake et al. (2011)].
We make the following predictions: (1) if the rostrosoma of the three groups evolved in common in all three groups, then we would expect the same morphology, no features missing, no additional features.. (2) If the rostrosoma evolved through parallelism, i.e., independent parallel evolution of homologous elements, we would expect finding subtle differences in the design of the rostrosoma and the involvement of different features, e.g., attachment of pharyngeal muscles. (3) If the rostrosoma evolved as a homoplastic element we would expect different morphological elements contributing to the rostrosoma.
The homologization of elements of the rostrosoma hinges on their topographic relationship and specific features that characterize them. The epistome and the labrum are the frontal sclerites of the prosoma. They may be separated by an external sulcus. Internally, the epistome is attachment site for the dorsal pharyngeal dilator muscles of the precerebral suction pump; the labrum has no intrinsic muscles (e.g., Bitsch and Bitsch 2007). The labrum overhangs the mouth; it develops from embryonal rudiments of limb-bud-like primordia (Kimm and Prpic 2006), while the epistome is part of the prosomal shield. Thus, the labrum is diagnosed as the apical, hemolymph-filled element overhanging the mouth opening. The epistome is the sclerite that serves as an attachment point for the dorsal pharyngeal dilator muscles of the precerebral suction pump. Labrum and epistome are elements of the ground pattern of chelicerates (Bitsch and Bitsch 2007) if not arthropods (Scholz and Edgecombe 2006; Haug and Rötzer 2018; Haug 2020).
The lateral lips of solpugids, pseudoscorpions and (some basal) Acari (Opilioacarida) supposedly originate as paired lobes from the pedipalpal coxae (gnathobasis, gnathocoxae). Characterization is by topography, with no additional specific features available. Medial lobes of the pedipalpal coxae occur also in other chelicerate clades where they are included as element of the feeding apparatus (Merostomata, Scorpiones, Uropygi, Amblypygi, Araneae; Börner 1902; Kästner 1925, 1931a; Aria and Caron 2017; Haug et al. 2019; Haug 2020). Medial lobes of the pedipalpal coxae are therefore considered an element of the chelicerate ground pattern (Howard et al. 2020). Consequently, our analysis needs to focus on specific features like modification of shape and formation of apodemes.
The pharyngeal muscular suction pump is an important functional element of the anterior intestinal tract and associated with the rostrosoma. The pharyngeal (= precerebral) suction pump and a postcerebral suction pumps can be found in Amblypygi (Kästner 1931b), Araneae (Felgenhauer 1999), Ricinulei (Ludwig et al. 1994; Talarico et al. 2011), Scorpiones (Farley 1999) and Thelyphonida (Kästner 1931b). The posterior suction pump is not present in Acari (Alberti and Coons 1999; Coons and Alberti 1999), Opiliones (Pinto-da-Rocha et al. 2007), Pseudoscorpiones (Weygoldt 1969) and Solifugae (Klann and Alberti 2010). Shultz (1990, 2007) coded the occurrence of a pre- and postcerebral suction pump in his extensive morphological data matrix (postcerebral pharynx in Shultz 1990). Despite the incompleteness of the data matrix, the suggestion emerges that the precerebral suction pump is a symplesiomorphy of chelicerates and the postcerebral suction pump may have evolved independently several times. The arrangement of pharyngeal musculature and their attachment points provide possible clues for the homologization of elements (epistome = attachment site of the dorsal pharyngeal dilator muscle). Diverging muscles morphology and different attachment sites of musculature are indicative of parallel or divergent evolution of the rostrosoma.
Materials and methods
Pseudoscorpiones
Histological series of a male and a female Neobisium carcinoides (Hermann, 1804) were available at the Department of Biology, University of Munich (LMU). The series were produced for an earlier study (Mehnert et al. 2018), but not analyzed for the rostrosoma. The material was historesin embedded, sectioned at 2 µm section thickness and stained with Rüdeberg stain (Rüdeberg 1967). The series are deposited at the Department of Biology and accessible upon request. For µCT-imaging, we used a series of images of a female Chernes hahnii (C.L. Koch, 1839) available at MorphoBank (O’Leary and Kaufman 2011; http//morphobank.org;project 2802). This series was also originally created by Mehnert et al. (2018).
Solifuges
Histological serial section of Oltacola gomezi Roewer, 1934, Eremobates durangonus Roewer, 1934, Eremobates pallipes Say, 1823 and Galeodes caspius Birula, 1890 were available on loan from the collection of the Zoological Institute at University of Greifswald. The histological series were originally created for a study by Klann (2009) and Klann and Alberti (2010). Material was paraffin embedded, sectioned at 4-10 µm section thickness and stained with Azan-Heidenhain or hematoxylin–eosin. A complete set of µCT-images from Galeodes granti Pocock, 1903 was created for an earlier study of the tracheal system (Franz-Guess et al. 2016) and is publicly available at MorphoBank (O'Leary and Kaufman 2012; http://morphobank.org/permalink/?P2422).
Acari/Parasitiformes
Numerous histological serial sections of Ixodes ricinus were available at the Department of Biology, University of Munich (LMU). The series were produced for an ongoing research project (Starck, unpubl. material), but not analyzed for the morphology of the mouthparts. The histological series are deposited at the Department of Biology, University of Munich (LMU) and accessible upon request. Material was epon embedded, sectioned a 1 µm section thickness and stained with Rüdeberg stain. µCT-images of Ixodes ricinus were available from Starck et al. (2018).
Microscopy
Histological images were taken either with an Olympus BX61VS microscope equipped with an automated scanner table, a X10 Camera and VS-ASW Vers. 2.9 software, or a Zeiss Axiophot equipped with an Axiocam ERc 5 s camera and Zen 2.3 (blue edition), Zeiss; details are given in the figure captions. When taking images with the Olympus microscope and VS-ASW software (all Solifugae, some Ixodes sections), we employed the extended focal imaging option to improve focal depth of relatively thick sections. All images were adjusted for tonal range; background was removed and labeling was added using Adobe Photoshop CS2 Vers.09, 1990–2005. Schematic drawings were created using Inkscape 1.0.2-2 (2021) and SketchBook Version 8.7.1—2019.
Results
Rostrosoma of Solifugae
The rostrosoma of Solifugae is located between the chelicerae and anterior to the coxae of the pedipalps (Fig. 1). It is an elongate, laterally compressed structure. At its anterior end, it forms a triangular upper lip and two lateral lips that carry numerous bristles. The mouth is a tiny, terminal opening (Figs. 1C, 2A, B) between upper and lateral lips. It extends slit-like along the anterior rostrosoma for a short distance to a point where the three lips merge. The rostrosoma is free for most of its length (Fig. 1A, B) but, more basally, the lateral parts of the rostrosoma fuse with the coxae of the pedipalps (Figs. 1A, B, E–H, 2D, E). Those lateral parts of the rostrosoma that are fused with the medial face of the pedipalpal coxae continue into two lateral apodemes (Fig. 2E, F). The dorsal, sclerotized part of the rostrosoma grades into the mediodorsal surface of the pedipalpal coxae and into the soft endocuticular membrane of the frontal face of the carapace (Fig. 3A, D).
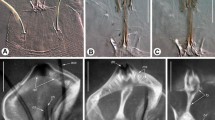
Galeodes granti, rostrosoma and mouth opening, µCT-image series. A 3D-reconstruction of the rostrosoma seen from front; chelicerae are half cut. The rostrosoma forms from the medial flanges of the pedipalpal coxae. B Same reconstruction, but rostrosoma shown in from a ventro-lateral view. The separation of the anterior rostrosoma into an upper lip and paired lateral lips is clearly seen. The distance between the lateral lips is probably artificially increased from drying of the specimen before scanning. C µCT-image, cross-section through the mouth opening. D µCT-image, cross-section through the anterior part of the rostrosoma, just behind the mouth opening. E µCT-image, cross-section through the rostrosoma at a position where it merges with the coxae of the pedipalps. F µCT-image, cross-section through the rostrosoma, which is completely merged with the pedipalpal coxae. G µCT-image, cross-section through the basis of the rostrosoma and the pedipalpal coxae. Only the dorsal cover of the rostrosoma is distinct, other parts have been internalized and function as apodemes. H µCT-image, cross-section through the anterior part of the prosoma. Proximal residues of the rostrosoma remain as cuticular elements surrounding the pharynx and function as apodemes. ap apodeme, Ch chelicera; Cp coxa of pedipalp; ll lateral lip; mo mouth opening; ph pharynx; Pp pedipalp; R rostrosoma; ul upper lip. Scale bar for images C-H at bottom of figure
Oltacola gomezi, histological cross-sections through the rostrosoma, from the mouth opening to the basis of the rostrosoma; Azan stain. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides were scanned with an UPlanSApo 20 × 0.75 objective. A Mouth opening at the anterior end of the rostrosoma. At this position, the mouth opening is between the upper lip and the paired lower lips; numerous bristles form a sieve that covers the mouth opening. B Section through the mouth opening at a slightly more basal position than (A), just before upper and lower lips merge to form the body of the rostrosoma. C Cross-section through the rostrosoma with the pharynx. D Cross-section through the rostrosoma at a position where it merges with the coxae of the pedipalps. E Cross section through the rostrosoma at a basal position where it is completely fused to the coxae of the pedipalps; the cuticle forming large apodemes for muscles in the rostrosoma. F Cross-section through the pharynx in the prosoma. The ventral sclerite that extends along the entire rostrosoma, can still be differentiated and is a topographically distinct skeletal element between the coxae of the pedipalps. add anterior bundle of the dorsal pharyngeal dilator muscle; ald anterior bundle of the lateral pharyngeal dilator muscle; br bristles covering the mouth opening; cm circular pharyngeal muscle; dpm dorsal pharyngeal dilator muscle; enc endocuticle; exc, exocuticle; hls hemolymph space; ldm lateral pharyngeal dilator muscle; ll lateral lip; mo mouth opening; ms membraneous septum between the hemolymphatic spaces in the ventral rostrosoma; n nerve; ph pharynx; Ppc coxa of pedipalp; scf soft cuticular folds of lateral lips; tr trachea; sul soft cuticle of upper lip; ul upper lip; vms ventralmedian sclerit; vrm ventral rostrosoma muscle; vs ventral slit between the lateral lips
Oltacola gomezi, histological cross-sections through the rostrosoma and the anterior part of the prosoma; Azan stain. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides were scanned with an UPlanSApo 20 × 0.75 objective. A Cross-section through the anterior prosoma at a position of the eyes. The rostrosoma is free and located between the chelicerae and the coxae of pedipalps. Note the soft cuticle covering the prosoma anterior and topographically ventral to the eyes (blue stain) while all other parts of the body are covered with sclerotized cuticle (orange stain). The frame indicates the detail of the rostrosoma shown in figure (B). B High power magnification of the rostrosoma (detail from A, frame). Note the topographically distinct differentiation/sclerotization of the cuticle covering the rostrosoma and lining the pharynx. C Cross-section through the rostrosoma few sections distant from (B) to document the connection of the pharyngeal pockets in (B) with the lumen of the pharynx. D Cross-section through the prosoma at a position where the rostrosoma merges with the pedipalpal coxae. See Fig. 3 for details of the rostrosoma. add anterior bundle of the dorsal pharyngeal dilator muscle; ald anterior bundle of the lateral pharyngeal dilator muscle; Ch chelicera; cm, circular pharyngeal muscle; E eye; enc endocuticle; exc exocuticle; hls hemolymph space; mc membraneous cuticle; ms membraneous septum between the hemolymphatic spaces in the ventral rostrosoma; ph pharynx; php pharyngeal pocket; Pp pedipalp; R rostrosoma; * artifact
The 3D-reconstruction of the rostrosoma from µCT-scans of Galeodes granti (Fig. 1A, B) shows a continuous connection of all parts of the rostrosoma with the coxae of the pedipalps. However, µCT-imaging does not render contrast between soft endocuticle and sclerotized exocuticle. Therefore, the cuticle of the rostrosoma is imaged as continuous with the coxae of the pedipalps and individual sclerites are not recognized. Images in Fig. 1C–H represent individual cross-sectional µCT-images from the tip (mouth opening) to the basis of the rostrosoma.
Routine histology (here: Azan-Heidenhain stain) provides details about the sclerotization of the cuticle because of the higher resolution and because of the different staining properties of soft endocuticle (collagen fibers in the endocuticle stain blue with Aniline blue) and sclerotized exocuticle (proteins in exocuticle stain red/orange with Azocarmin). The differential staining of endocuticle and exocuticle is important because it allows to determine soft borders between sclerites. The cuticle between sclerites is a non-sclerotized procuticle and, therefore, stains blue, while the cuticle of sclerites consists of a thin endocuticle and a thick sclerotized exocuticle (stains red/orange). Thus, histology and appropriate staining add information about the topographic borders of individual sclerites. Because soft cuticle between sclerites also allows for movements of sclerites against each other, functional interpretations become possible.
Cross-sections through the mouth opening at the apical tip of the rostrosoma show the folloeing three elements: a dorsal lip and two lateral lips (Fig. 2A, B). The dorsal lip is an almost triangular element consisting of an intensively sclerotized dorsal part and a soft ventral part. At this apical position of the rostrosoma, the dorsal lip contains few muscle fibers, hemolymphatic space and a few tracheae. The muscle fibers are part of the pharyngeal suction pump (anterior bundle of the dorsal pharyngeal dilator muscle; see below).
The cuticle of the two lateral lips is much thinner as compared to the dorsal lip (Fig. 2A, B). In particular, the upper, inner and ventro-lateral flanges consist predominantly of soft endocuticle (stained blue with Azan Heidenhain), which is superficially covered by a thin layer of exocuticle (stained red with Azan-Heidenhain). Each lateral lip contains a large hemolymphatic space and few muscle fibers. Lateral lips and dorsal lip remain separated for a short distance, thus forming one ventral and two lateral slits. More basal along the rostrosoma, the lateral lips and the dorsal lip merge forming the body of the rostrosoma. The ventral suture between the fused lateral lips is intensively sclerotized and remains separated from the surrounding cuticle by soft cuticle (Fig. 2C, D); muscle fibers attach to this sclerotized cuticle in the ventral midline (Figs. 2C, D, 3D). This zone represents a distinct and elongate sclerite, separated by soft endocuticle from the lateral and dorsal parts of the rostrosoma [= postoral sternapophysis in Shultz (2007); tritosternum in Kästner (1931b), or labium in van der Hammen (1989)]. Screening through all serial sections shows that this sclerite extends from a position where the lateral lips merge to a position between the coxae of the pedipalps and ultimately connects to the apodeme formed between the coxae of the first walking leg.
The cross-sectional anatomy of the rostrosoma changes from tip to basis, but a continuous seam of thick endocuticle (Figs. 2C, D) joins the upper and lateral lips, on each side of the rostrosoma. It ends at a position where the rostrosoma merges with the coxae of the pedipalps (Fig. 2D, E). We recognize the following three sclerites of the rostrosoma: (i) a dorsal sclerite, (ii) lateral flanges that continue apically into the lateral lips and merge basally with the pedipalpal coxae and (iii) the ventral median sclerite that connects to the intercoxal apodeme of the first walking leg.
Pharynx: Central in the rostrosoma, behind the mouth opening, lies the pharynx. In the µCT-images, it is roughly Y-shaped (Fig. 1D–H). In all histological slides, it is wide open, probably because contraction of the dilator muscles during the fixation process (Figs. 2C–H, 3). The pharynx is internally lined by cuticle, which differs regionally in the degree of sclerotization, in cross section as well as along the rostrosoma. In an anterior cross-section (Figs. 2C, 3A–C), the cuticle of the lateral flanges of the pharynx is sclerotized while it is soft in the dorsal (roof) and the ventral tip. Soft cuticular pouches open lateral from the ventral region part of the pharynx (Fig. 3B, C). More basal along the rostrosoma (Figs. 2D, E, 3D), the cuticular lining of the pharynx is more completely sclerotized, in cross-section only narrow dorso-lateral and ventral sectors are soft; those extend as thin bands along the rostrosoma.
Rostrosoma muscles: The pharyngeal suction pump of Solifugae is located in the rostrosoma. Because of the three-dimensional orientation of the muscle fibers, at least two sectional planes are necessary to fully document the muscular suction pump (Figs. 2, 3, 4). The extensively developed pharyngeal muscular system consists of dorsal dilator muscles, lateral dilator muscles and circular constrictor muscles. The topography of the muscle attachment suggests that these muscles act directly as a suction pump. Additionally, we found indirect muscles that might support pumping movements of the rostrosoma.
Oltacola gomezi, histological sections in longitudinal and horizontal orientation through the prosoma of a juvenile individual. Images A-D proceed from ventral to dorsal; hematoxylin–eosin stain. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides were scanned with an UPlanSApo 20 × 0.75 objective. A Ventral section plane. The two portions of the ventral rostrosoma musculature that extend from the ventral medial sclerite to the lateral wall of the rostrosoma are clearly distinguished in this section. B Slightly more dorsal than (A), this image shows a horizontal section through the pharynx in the rostrosoma. The pharyngeal suction pump, i.e., lateral dilator muscles and circular constrictor muscles, is divided by the posterior portion of the ventral rostrosoma muscle into the anterior and posterior part of the precerebral suction pump. The anterior portion of the lateral dilator muscle takes an oblique course and inserts at the anterior tip of the pharynx (compare with cross-sections in Fig. 3C, D). C Dorsal section through the rostrosoma showing the topography of the dorsal dilator muscles. Note that the dorsal dilator muscles take a straight dorso-ventral course (this shows up in cross-section) for most of the rostrosoma. Only the anterior portion of the dorsal dilator muscles takes a more oblique course and inserts at the anterior tip of the rostrosoma. D Dorsal horizontal section through the prosoma and rostrosoma. Only cross-sections of the dorsal dilator muscles are seen. bR basis rostrosomae; cb cuticular bridge (at end of epistome); Ch Chelicera; cm circular pharyngeal muscle; cht cheliceral teeth; dpm dorsal pharyngeal dilator muscle; L1–L3, walking legs 1–3; ldm lateral pharyngeal dilator muscle; n1 nerve of walking leg 1; ph pharynx; syc syncerebrum; syn synganglion; vrm1, 2 ventral rostrosoma muscle portion 1 and 2, respectively
Constrictor muscles are serially arranged muscles around the pharynx. They interchange with attachment sites for the dorsal and lateral dilator muscles. The constrictor muscles are actually not circular muscles but longitudinal muscles that attach at the tips of the lateral and ventral arms of the Y-shaped pharynx (Figs. 2C–F, 3). They are arranged serially, alternating with the lateral dilator muscles and extend from the basis of the pharynx (just in front of the brain; Fig. 4B) to about the anterior third of the rostrosoma. They are not found in the apical third of the rostrosoma.
Lateral dilator muscles are found in two packages, one basal package and an anterior package (Fig. 4B). They are interrupted by large ventral muscles of the indirect musculature of the rostrosoma (Fig. 4A, B). The lateral dilator muscles attach to the pharynx and, basally, to the apodemes of the pedipalpal coxa (Fig. 4A , B). More apically, they extend between the pharynx and the lateral wall of the rostrosoma (Fig. 2C, D). In the anterior part of the rostrosoma, the lateral pharyngeal dilator muscles span in a wide angle between the pharynx and the anterior tip of the rostrosoma. The most anterior muscle fibers take a steep inclination angle, extend almost parallel with the pharynx and attach to the tip of the rostrosoma (Fig. 4B). Thus they appear in cross-section (Fig. 2B–E).
The dorsal pharyngeal dilator muscles extend between the pharynx and the dorsal cover of the rostrosoma (epistome; Fig. 2C–E). They extend along the entire length of the rostrosoma, from the basis of the pharynx to the tip of the rostrosoma (Fig. 4C, D). No ventral dilator muscles were found.
Rostrosoma of Pseudoscorpiones
The rostrosoma of pseudoscorpions is elongate and laterally compressed. It is enclosed between the laminae inferiores of the pedipalpal coxae and the chelicerae (Fig. 5). The laminae inferiores of the pedipalps (= lateral lips) interlock at their medio-ventral border thus forming a ventrally closed space around the rostrosoma. The chelicerae close that space dorsally. The enclosure of the rostrosoma reaches until up to its anterior tip (Figs. 5, 6). The rostrosoma consists of a dorsal part (upper lip; taphrognath in Chamberlin 1931; Weygoldt 1969) and a somewhat shorter ventral part, i.e., lower lip (lophognath in Chamberlin 1931; labium in van der Hammen 1989; lower lip in Weygoldt 1965, 19681969, 1971). The upper lip has ventro-lateral flanges that embrace the lower lip (Figs. 6B, C, 7A, B). Between upper lip and lower lip is the mouth opening, which actually consists of an anterior opening and two elongate lateral slits. At the basis of the rostrosoma, the lateral wall of the upper lip merges with the bases of the pedipalps forming a strongly sclerotized apodeme (Fig. 7A, B). From that point on, the dorsal cover of the rostrosoma extends as an individual sclerotized element (apodeme) into the prosoma. It provides the attachment site for the dorsal pharyngeal dilator muscles into the prosoma [= internalized epistome; intermaxillary jugum in Beier (1931) and Chamberlin (1931); Oberlippenapodem in Weygoldt (1965); Figs. 5N, 7A, B, 8].
Chernes hahnii, µCT-series through the rostrosoma from anterior to posterior. A–N Consecutive, equidistant cross-sectional µCT-images. All images same size (scale bar in M). Ch chelicera; dpm dorsal pharyngeal dilator muscle; eps epistome; ldm lateral pharyngeal dilator muscle; li lamina inferior; ll lateral lip (endite of pedipalp); mcl musculus compressor labri; ph pharynx; Pp pedipalp; Ppc coxa of pedipalp; R rostrosoma; ul upper lip; vl ventral lip
Neobisium carcinoides, semi-thin cross sections (2 µm section thickness) of a female. Hydroxyethyl methacrylate (Historesin; Leica Microsystems, Wetzlar, Germany) embedded material. Rüdeberg stain. Images were taken with a Zeiss Axiophot equipped with a Plan-Neofluar 20x/0.50 or a Plan-Apochromat 63x/1.4 oil immersion lens and an Axiocam ERc 5 s camera. Multiple images were stitched using Image Composite Editor Vers. 2.9 (Microsoft). A Cross-section through the anterior tip of the rostrosoma. The rostrosoma is free between the chelicerae (dorsal) and the lateral lips (= endites of the pedipalps). At this position, the tip of the rostrosoma is hemolymph filled and contains no muscles (= labrum). B Cross-section through the rostrosoma at a little bit more basal position. The pedipalps are ventrally interlocked at their medio-ventral edge (= lamina inferior) and dorsally abutting against the rostrosoma (lamina superior), thus forming a preoral cavity. However, in the histological sections, the pedipalps are slightly displaced (indicated by *). The black frame indicates the part shown in (B′) at higher magnification. B′ Detail of (B). C Section little further basal to the previous section. The rostrosoma is dorsally merged with the dorso-medial edge of the pedipalps (= lamina superior). Ventrally, the pedipalps are interlocked by the lamina inferiores. The space between the rostrosoma and the pedipalps is the perioral cavity. The black frame indicates the part shown in (C′) at higher magnification. D Detail of (C) showing a cross section through upper and lower lip. Ch chelicera; cp cuticular pad of rostrosoma; dpm dorsal pharyngeal dilator muscle; hls hemolymph space; li lamina inferior; ll lateral lip; lfu lateral flanges of upper lip; li lamina inferior; ls lamina superior; mcl musculus compressor labri; ls lamina superior; Ppc coxa of pedipalp; ul upper lip; vl ventral lip
Neobisium carcinoides, semi-thin cross sections (2 µm section thickness) of a female. Hydroxyethyl methacrylate (Historesin; Leica Microsystems, Wetzlar, Germany) embedded material. Rüdeberg stain (Rüdeberg 1967). Images were taken with a Zeiss Axiophot equipped with a Plan-Neofluar 20x/0.50 or a Plan-Apochromat 63x/1.4 oil immersion lens and an Axiocam ERc 5 s camera. Multiple images were stitched using Image Composite Editor Vers. 2.9 (Microsoft). A Cross-section through the rostrosoma at a position where the lateral wall of the upper lip has been completely merged with the medial sides of the pedipalps. The dorsal face of the rostrosoma (epistome) is continues into the prosoma like an apodeme for the dorsal pharyngeal dilator muscle. The lower lip has a rhombic cross-section and is free between the bases of the pedipalps. A large hemolymphatic space fills the lower lip. B Neighboring section to (A) in higher magnification. C Cross-section through the prosoma at the level of bases of pedipalps and chelicerae. At that position, upper and lower lip of the rostrosoma are fused with the basis of the pedipalps. D Detail of (C) as marked by the rectangle in (C). E Cross-section through the prosoma at the level of the pharyngeal pump. ap apodeme; Ch chelicera; cp cuticular pad of rostrosoma; dpm dorsal pharyngeal dilator muscle; eps epistome; hls hemolymph space; lfu lateral flanges of upper lip; li lamina inferior; ldm lateral pharyngeal dilator muscle; lrm lateral rostrosoma muscle; ls lamina superior; mo “mouth opening”; Pp pedipalp; ul upper lip; vl ventral lip;
Neobisium carcinoides, semi-thin longitudinal sections (2 µm section thickness) of a male. Hydroxyethyl methacrylate (Historesin; Leica Microsystems, Wetzlar, Germany) embedded material. Rüderberg stain (Rüdeberg 1967). Images were taken with a Zeiss Axiophot equipped with a Plan-Neofluar 20x/0.50 lens and an Axiocam ERc 5 s camera. Multiple images were stitched using Image Composite Editor Vers. 2.9 (Microsoft). A Parasagittal longitudinal section through the rostrosoma and the synganglion. Labrum and internalized epistome are clearly characterized by the hemolymph space and the doweal pharyngeal dilator muscle, respectively. B Neighboring section to (A) at slightly enlarged magnification. Characteristic features of the upper lip, i.e., labrum, musculus compressor labri, dorsal pharyngeal compressor muscle and the internalized epistome are shown. C1 coxa of walking leg 1; cp cuticular pad of rostrosoma; dpm dorsal pharyngeal dilator muscle; eps epistome; hslb hemolymph sinus in labrum; lb labrum; mcl transversal rostrosoma muscle; Ppc coxa of pedipalp; syn synganglion; * artifact
The lower lip is thin and laterally compressed at the anterior tip of the rostrosoma, but widens to the basis attaining a rhombic cross-sectional shape (Figs. 6, 7). It merges to the coxae of the pedipalps, but a little bit more basal than the upper lip. There, it forms an intensively sclerotized cuticular bridge that fuses with the apodemes formed by the dorsal lip (Fig. 7C, D). The pharynx begins at this basal position. The anterior and dorsal lining of the pharynx is characterized by a thick cuticular pad (Figs. 7C, D, 8), that is as an attachment point for the dorsal pharyngeal dilator muscles. The ventral side of the anterior pharynx is stabilized by the cuticular bridge and is probably not movable. The middle part of the pharynx is characterized by an X-shaped cross-section that is attachment for extensively developed dilator muscles (Fig. 7E).
Sections of the pseudoscorpions were stained with Rüdeberg stain (Rüdeberg, 1967); thus soft cuticle is pale, light blue. Sclerotized cuticle stains intensively blue. The cuticle of the upper lip is relatively homogenous sclerotized except for the tip of the rostrosoma and the inner side of the ventral flanges, which are soft. The roof of the groove between the flanges is intensively sclerotized along the length of the rostrosoma and serves as attachment for dorsal pharyngeal dilator muscles. The epidermal epithelium at the tip of the rostrosoma appears modified, but the resolution in light microscopy is insufficient for a cytological analysis. The lower lip has an intensively sclerotized upper sector, but the lateral and ventral sides are soft. The lateral lips and the laminae inferiores of the pedipalpal coxae are characterized by a thick endocuticle that is covered by a thin layer of exocuticle and are distinctly different for the remaining cuticle of the pedipalpal coxae.
Rostrosoma muscles: The precerebral pharyngeal suction pump is well developed. It is located at the basis of the rostrosoma and in the anterior prosoma. The anterior tip of the rostrosoma contains an extensive hemolymphatic space (Figs. 6A, 8), but no muscles. Located between this hemolymphatic space and the pharyngeal suction pump is a prominent transversal muscle (Figs. 6B, C, 8; M. compressor labri in Chamberlain 1931 and Weygoldt 1965). The ventrolateral flanges of the upper lip that embrace the lower lip have no intrinsic musculature. The lower lip has no intrinsic muscles but a large hemolymphatic space.
The pharyngeal suction pump consists of large, dorsal pharyngeal dilator muscle (taphrognath levator muscle in Chamberlain 1931), strong lateral dilator muscles and constrictor muscles. The dorsal pharyngeal dilator muscle spans between the cuticular pad of the pharynx and the (internalized) epistome (Figs. 7, 8). The lateral dilator muscles span between the lateral wall of the pharynx and the lateral wall of the pedipalpal basis (Fig. 7E). The constrictor muscles are short, straight muscles extending between the two upper and two lower arms, respectively, of the X-shaped pharynx (Fig. 7D). No ventral dilator muscles were found.
The longitudinal sections in Fig. 8 document the extension of the dorsal pharyngeal dilator muscle and that its attachment, i.e., the epistome, is internalized into the prosoma forming an apodeme. It also documents, that there are no ventral muscles associated with the pharynx of pseudoscorpions. The pharynx is relatively short and continues into the oesophagus, which is surrounded by the synganglion.
Mouthparts of Ixodes ricinus (Acari/Parasitiformes)
Mouthparts of Ixodes ricinus are on the capitulum (= gnathosoma; Figs. 9, 10A, 11B, C), i.e., the anterior acarine body region that is functionally specialized for feeding. They comprise the chelicerae, pedipalps, epistome, labrum and the hypostome. The basis capituli forms a solid ring of sclerotized cuticle that encloses all mouthparts, mouth opening and pharynx and is the articulation point for the pedipalps (Fig. 9K–N). The lower anatomical region of the basis capituli (infracapitulum sensu van der Hammen 1983) carries the hypostome (lateral lips sensu van der Hammen 1983) and the palps and contains the mouth opening and the pharynx.
Ixodes ricinus, µCT-image series of equidistant horizontal images through an individual. The mouthparts are oriented ventrad related to the anterior–posterior axis of the animal (see inset in A, B row). Sections A–H are shown as cross-sections while the following images grade into a horizontal sectioning plane. Ch chelicera; Hy hypostome; ic infracapitulum; ldm lateral pharyngeal dilator muscles; ph pharynx; Pp pedipalp; Ppc coxa of pedipalp; inset not to scale
Ixodes ricinus, preoral canal, mouth opening and pharyngeal valve in sagittal and parasagittal section. Selected images from a series of semithin sections through the capitulum of a fasting male; epon embedded material, all sections 1 µm, Ruedeberg stain. In all sections, anterior is to the left side. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides scanned with a UPlanSApo 40 × 0.95 objective. A Low power magnification for overview and topographic orientation. The rectangle indicates the position of the high power magnification images in “B” and “C”. The section in “A” is a neighboring section to B and C from the same series. B Sagittal section through the preoral canal, mouth opening and pharynx. The membrane covering the hypostomal groove is clearly distinct from the hypostome. The hypostomal groove forms an extension at the base of the hypostome, just in anterior to the mouth opening / tip of labrum. The labrum covers the mouth opening. It has a distinct thickening of the cuticle on its ventral side (see also cross-sections in Fig. 12), which forms a plug that appears to fit into the slit-like mouth opening. The labrum contains a hemolymph sinus, which is continuous with the hemolymph space of the capitulum. Anterior dilator muscles insert on the ventral side of the pharynx. C Parasagittal section through the preoral canal, mouth opening and pharynx. The membrane covering the hypostomal groove and groove extension merges to the cuticle of the mouth opening. A thin cuticular tip of the labrum reaches a short distance into the preoral canal. The section documents the lateral wings of the labrum that extend along the downward bending pharynx and form attachment sites muscles that attach to the floor of the salivarium. adm anterior dilator muscles of pharynx; bc basis capitulum; Ch chelicera; Chs cheliceral sheath; dpm dorsal pharyngeal dilator muscle; ep epistome; exhc extension of hypostomal groove; hslb hemolymph sinus in labrum; Hy hypostome, lb labrum; lwlb lateral wing of labrum; mc membrane covering hypostomal groove; ph pharynx; poc preoral canal; Pp pedipalp; sal salivarium
Ixodes ricinus, microscopic anatomy of the pharynx. The pharynx is part of the ectodermal intestinal system and lined by cuticle. It is the place of the powerful precerebral suction pump. Selected images from different series of semithin sections through the capitulum of fasting males; epon embedded specimen, all sections 1 µm, Ruedeberg stain. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides scanned with a UPlanSApo 40 × 0.95 objective. A Cross-section through the pharyngeal valve, the alae of the labrum bend lateral and ventral providing large insertion areas for the anterior dilator muscles and the muscles below the salivarium. B Horizontal section through the capitulum and anterior part of the idiosoma of a fasting male. The section documents two distinct groups of dilator muscles attaching to the pharynx, i.e., the anterior dilator muscles and the posterior lateral dilator muscles. Circular muscles interrupt the group of lateral dilator muscles. C Parallel section to “B”. This section also shows the anterior tip of the labrum. D Cross-section through the basis capituli, showing the X-shaped cross section of the pharynx with lateral and circular musculature attaching. A thick cuticle internally lines the pharynx. adm anterior dilator muscles; bc basis capituli; Ch chelicerae; lbp labral plate; ldm lateral dilator muscles of pharynx; lwlb lateral wing of labrum (ala); ms muscle between salivarium and pharynx; ph pharynx; pht pharyngeal teeth; Pp pedipalp; sal salivarium; sChp subcheliceral plate; syn syncerebrum; tlb tip of labrum
In Ixodidae, the hypostome is the ventral extension of the infracapitulum. It consists of two elongate elements (lateral lips sensu van der Hammen 1983) that are fused along their midline and carry hooks on their sides (Figs. 9, 10, 11). The dorsal side of the hypostome has a V-shaped channel extending from the tip to its basis. A thin cuticular membrane covers this channel, which is filled with hemolymph. At the basis of the hypostome, the channel connects through lateral arms with the hemolymph sinus in the labrum (Fig. 12).
Ixodes ricinus, series of cross-sectional histological images through the preoral canal and the anterior pharynx documenting the morphology of the labrum and the pharyngeal valve; the sequence of images is from apical to basal along the hypostome. Epon embedded specimen, all sections 1 µm, Ruedeberg stain. All images were taken with a Zeiss Plan-Apochromat 63 × oil immersion objective on a Zeiss Axiophot equipped with an Axiocam ERc 5 s. Same magnification applies to all images, scale bar in “H”. A Cross-section through the basis of the hypostome. The hypostomal gutter in the midline of the hypostome is a hemolymph-filled canal that is covered by a thin cuticular membrane with a median thickening. B Section through the tip of the labrum. C. A few microns more basal along the hypostome, a thin cuticular membrane connects the labrum laterally to the hypostomal groove. The membrane separates the preoral canal into the dorsal salivarium and the ventral food canal. The hypostomal gutter is parting into two lateral wings. The hemolymph-filled lumen of these wings is in open contact with the lumen of the hemolymph sinus in the labrum (see figures D, E and F). D This image documents the ending of the hypostomal gutter and its parting into two lateral wings that connect the lumen of the hypostomal gutter with the hemolymph sinus in the labrum. Between the two wings of the hypostomal canal, slit shaped opening occurs which continues into the mouth opening/pharyngeal opening. E The lateral wings of the hypostomal gutter have completely merged to the lateral wall of the mouth opening. The labrum contains a hemolymph sinus which is in open connection with the hypostomal gutter and with the hemolymph spaces in the basis capituli. The roof of the labrum is a thin cuticular membrane that bulges into the salivarium. The center of the labrum contains a solid cuticular rod (continues into the alae). On its ventral surface it carries a pointed ridge that appears to fit into the slit shaped mouth opening. F Section a few microns distant to “E”, documenting same morphology. G Cross-section through the anterior part of the pharyngeal valve, where the cuticular rod of the labrum just widens into the alae. The lateral wall of the pharynx shows a cuticular thickening. H Cross-section through the pharyngeal valve. The labrum is a thick plate (which continues into the alae. On the ventral side the labrum has a pointed ridge that reaches into the mouth opening/anterior pharynx. The lateral wall of the pharynx has two distinct cuticular teeth. The microscopic anatomy suggests that the ventral ridge of the labrum and the pharyngeal teeth might meet when hemolymph pressure in the labrum is increasing, thus might act as closing mechanism for the mouth opening/pharyngeal opening. adm anterior dilator muscles of pharynx; bsal bottom of salivarium = roof of labrum; cclb connection between hypostomal canal and hemolymph space in labrum; Ch chelicera; Chf fixed finger of chelicera; Chm movable finger of chelicera; Chs cheliceral sheath; clb cuticular core of labrum; fc food canal; hslb hemolymph sinus in labrum; Hy hypostome; lb labrum; lbp labral plate; lbm labrum membrane; lwlg lateral wing of v-shaped groove; lwlb lateral wing (alae) of labrum; mc membrane covering hypostomal groove; mmc medial thickening of mc; ms muscle between salivarium and pharynx; oChs outer cheliceral sheath; pho pharyngeal opening; pht pharyngeal teeth; phw pharyngeal wall; poc preoral canal; Pp pedipalp; sal salivarium; salc salivary canal; sChp subcheliceral plate; te tectum (tegulum); tlb tip of labrum; vmg v-shaped median groove in hypostome (hypostomal gutter); vrlb ventral ridge of labrum; vs ventral slit = > mouth opening;
Preoral canal
The adult preoral canal is formed between the hypostome on the ventral side and the paired chelicerae/cheliceral sheath on its dorsal side. It extends from the tip of the hypostome to the mouth opening at the basis of the hypostome (Figs. 9, 10, 11, 12, 13). The preoral canal is laterally open for almost the entire length of the hypostome.
Ixodes ricinus, microscopic anatomy of the pharynx muscles. A, B Semithin sections through the capitulum; epon embedded specimen, sections 1 µm, Ruedeberg stain. Images were taken with an Olympus BX61VS microscope equipped with a X10 Camera and VS-ASW Vers. 2.9 software. Slides scanned with a UPlanSApo 40 × 0.95 objective. C–F Epon embedded specimen, all Sects. 1 µm, Ruedeberg stain. All images were taken with a Zeiss Plan-Apochromat 63 × oil immersion objective on a Zeiss Axiophot equipped with an Axiocam ERc 5 s. A Parasagittal longitudinal section through the hypostome and capitulum of an adult male. This section documents all major pharyngeal muscles, specifically the anterior ventral pharyngeal muscles, the ventral dialator muscles, dorsal pharyngeal dilator muscles and lateral pharyngeal dilator muscles. B Horizontal section through the pharynx of an adult male tick. The section documents the lateral dilator muscles and the pharyngeal constrictor muscles. Both muscle packages alternate along the pharynx. C High power magnification of a cross-section through the pharynx of a female. The section documents the pharyngeal constrictor muscles that extend between the tips of the pharyngeal arms. D Neighboring section to (C) but now showing the attachment of the lateral dilator muscles (same individual). E Cross-section through the pharynx of a male. The section shows constrictor muscles and the attachment of the lateral dilator muscles. F Neighboring section to (E) showing the dorsal and ventral components of the constrictor muscles. The constrictor muscles constitute a complete muscular ring around the pharynx with attachment sites at the tips of the pharynx arms. adm anterior dilator muscles of pharynx; Chm cheliceral muscles; dpdm dorsal pharyngeal dilator muscle; go Gene’s organ; Hy hypostome; ldm lateral dilator muscles; pcm pharyngeal constrictor muscles; ph pharynx; sal, poc preoral canal; salivarium; syng; synganglion; vdm ventral dilator muscles
Mouth opening, pharyngeal valve
The mouth opening is located at the basis of the hypostome. The labrum covers the mouth opening dorsally. It is a thin, tongue-like structure extending from the epistome into the preoral canal (Fig. 10B, C, 12B, C). The labrum is hollow and hemolymph filled. On its ventral side, it forms a pointed edge that fits into the slit-like mouth opening. From the point where the labrum laterally connects to the hypostome (Fig. 12C–E), the preoral canal separates into the dorsal salivarium and the ventral pharynx.
The labrum has a complex three-dimensional shape. With its upper side, it forms the bottom of the salivary canal. It continues, without an externally visible border, into the epistome (characterized by the dorsal pharyngeal dilator muscle). Its ventral side forms the upper roof of the mouth and the anterior pharynx. The tip of the labrum is solid cuticular. The body of the labrum is hemolymph-filled. On its ventral side the cuticle forms a solid cuticular rod with a ventral ridge (Fig. 12B–F). This cuticular rod broadens and continues into the labral plate (Figs. 11A, 12G, H); the cuticular ridge on the ventral side of the labrum reaches into the mouth opening. The labral plate continues into lateral wings of the labrum (alae), which reach ventrad along the lateral wall of the pharynx and contribute to the pharyngeal valve (Fig. 10C).
The cuticular lining of the anterior pharynx carries a pair of lateral pharyngeal teeth (Fig. 12H) that, together with the ventral ridge of the labrum, constitute the pharyngeal valve. The shape of the cuticular teeth and the shape of the ventral side of the labrum suggest that they, together, tightly close the mouth opening, when the labrum moves down between the pharyngeal teeth (Fig. 12H).
Pharyngeal suction pump, salivarium
The pharynx begins behind the mouth opening and connects to the esophagus. It is internally lined by cuticle. In cross-section, the shape of the lumen of the pharynx varies between Y-shaped (anteriorly; Figs. 11A, 12F–H) to more complex with several lateral folds (posteriorly; Fig. 13D–F). The anterior part of the pharynx, immediately behind the mouth opening, bends ventrad (Figs. 10B, C, 13A). Several groups of dilator muscles and constrictor muscles attach to it forming a precerebral suction pump.
The anterior ventral dilator muscles of the pharynx extend between the floor of the mouth opening/beginning of pharynx to the basis capituli and probably widen the anterior part of the pharynx. The actual pharyngeal suction pump is located behind the lateral wings of the labrum (alae) where the pharynx takes a horizontal course. It consists of the dorsal pharyngeal dilator muscle, lateral dilator muscles, ventral dilator muscles and circular constrictor muscles (Figs. 10B, C, 13). The dorsal pharyngeal dilator muscles extend between the epistome and the dorsal pharynx wall (Figs. 10B, C, 13A). The lateral dilator muscles extend between the sides of the pharynx and the basis capituli (Fig. 11B, C, 13B); they alternate with circular constrictor muscles (Figs. 13C–F) that are arranged around the pharynx. Ventral pharyngeal dilator muscles (Fig. 13A) extend between the bottom of the pharynx and the basis capituli.
The salivarium is an important functional component of the feeding apparatus through which saliva from the salivary glands is delivered into the preoral canal. The ventral floor of the salivarium is formed by the dorsal sides of the epistome (basal) and the labrum (apical); the subcheliceral plate forms the roof of the salivarium. The subcheliceral plate has lateral extensions (“cervix” sensu van der Hammen 1983). Large lateral muscles, i.e., lateral muscles of salivarium (Vancova et al. 2020) originate from the salivarium and the lateral wings of the subcheliceral plate and insert to the side of the capitulum (Figs. 10B, C, 11B).
Discussion
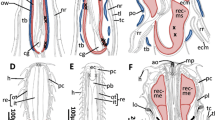
The goal of this study is to analyze the microscopic anatomy of the rostrosoma of Solifugae, Pseudoscorpiones and Acari/Parasitiformes and provide evidence for possible homologies of elements of the mouthparts. We focus on three elements, the epistomo-labral plate, the lateral lips of the rostrosoma and the musculature of the precerebral pharyngeal suction pump. A simplified schematic of the morphology of the rostrosoma in the three groups that allows for a straightforward comparison of the key elements discussed here is presented in Fig. 14.
Highly schematized and simplified drawings of the mouth parts of the three taxa discussed. Same body parts are labeled with same color to allow for simplified comparisons. Blue = epistome, yellow = labrum; pink, pharynx and associated musculature (in red). Drawings are not to scale. A Solifugae, B Pseudoscorpiones, C Parasitiformes. adm anterior dilator muscles; C1 coxa of first walking leg; Ch Chelicera (basis); Chs cheliceral sheeth; ecp endite of pedipalpal coxa; dpdm dorsal pharyngeal dilator muscle; eps epistome; hls hemolymph space; iss intercoxal sternal sclerite; lb labrum; lwlb lateral wing of labrum; li lamina inferior; mcl transversal rostrosoma muscle; ph pharynx; syng synganglion; vdm ventral dilator muscles; vl ventral lip; vrm ventral rostrosoma muscle
Epistome and labrum
According to Snodgrass (1948) and Bitsch and Bitsch (2007), the epistome and labrum are symplesiomorphies that chelicerates share with the ground pattern of arthropods. A recent analysis of fossil chelicerates recognized the labrum (“hypostome” in her terminology) as an element of the ground pattern of Neochelicerata (thus is missing in Pycnogonida and some fossil branches of Euchelicerata, but present in Xiphosuridae and Arachnida; Haug 2020). The border between the epistome and labrum is externally defined by a cuticular line and internally by the dorsal pharyngeal dilator muscles spanning between the epistome and the pharynx, while the labrum is hemolymph filled and has no intrinsic muscles (Snodgrass 1948; Bitsch and Bitsch 2007). The labrum overhangs the mouth opening, functionally serving as an “upper lip” (probably being moved by changing hemolymph pressure).
In solifuges, the dorsal side of the rostrosoma has no suture that could serve as external demarcation line between the epistome and labrum; therefore, Dunlop (2000) considered it an epistomo-labral plate. Histology shows that the entire dorsal face of the rostrosoma is the attachment site for the dorsal pharyngeal dilator muscles. The actual mouth opening of solifuges is at the tip of the rostrosoma, and no hemolymph filled structure overhangs it dorsally. Therefore, we suggest that the epistome constitutes the entire dorsal face of the rostrosoma and that the labrum is reduced. This interpretation (labrum reduced) is more parsimonious (one transformation) than a possible alternative interpretation, i.e., the muscle attachment of the dorsal pharyngeal dilator muscle expanded its insertion into the labrum and reduced the mouth-overhanging part of the labrum (2 modifications).—The basis of the epistome in solifuges continues into the soft frons of the prosoma, laterally it merges with the coxae of the pedipalps, as does the lateral parts of the rostrosoma (Fig. 14). This lack of external distinction is probably the reason why Shultz (1990) considered the (dorsal) rostrosoma a structure that develops from the “labrum” merging with the pedipalpal coxae, but Shultz (2007) described it as derived from the “epistome”. Our data now solve this ambiguity by showing that the dorsal rostrosoma of solifuges is built exclusively from the epistome and does not contain a labrum.
In pseudoscorpions, the dorsal surface of the rostrosoma is also smooth with no recognizable suture between the epistome and labrum. The anterior part of the upper lip of the rostrosoma overhangs the mouth opening, is hemolymph filled and has no muscles (see also Weygoldt (1964, 1965, 1968, 1969, 1971)). This is the labrum. The more basal part is the attachment site for the large dorsal pharyngeal dilator muscle, it is internalized into the prosoma forming an apodeme. In our morphological interpretation (Fig. 14) it represents an internalized epistome. Along the ventral side of the rostrosoma are two lateral flanges, which embrace the lower lip. These lateral flanges do not merge with the cuticle of the pedipalps, rather continue into the dorsal plate of the pharynx. The lateral flanges are unique elements of the rostrosoma of pseudoscorpions.
In ticks (and other Parasitiformes and Acariformes), the epistome and the labrum are delicate but distinct structures that cover the mouth and contribute to the formation of the salivarium. There is no external suture recognizable. However, the epistome is distinct as an attachment site for the dorsal pharyngeal dilator muscle. The labrum is a hemolymph filled “tongue” overhanging the mouth opening without muscles attaching to it (Fig. 14). The cuticle of the labrum forms a complex three-dimensional structure, i.e., labral plate and labral wings, which are part of the pharyngeal valve (a derived feature of ixodid ticks). Despite the bewildering morphological diversity of their mouth parts, a labrum and an epistome can be recognized in many acariforms and parasitiforms (e.g., van der Hammen 1983; Di Palma et al. 2006; 2009; Filimonova and Mironov 2010; Alberti et al. 2011; Shatrov 2011; 2012; Alberti and Dabert 2012).
The epistome, labrum and dorsal pharyngeal dilator muscle are symplesiomorphic elements of the chelicerate ground pattern, but their specific morphology is derived. Therefore, we consider the epistome–labrum complex in ticks independently derived from a plesiomorphic pattern. As pointed out by Dunlop (2000), it is homologous with the epistome–labrum in pseudoscorpions and the epistome is homologous with the epistome of Solifugae, but the specific morphologies of these elements in the three groups suggest independent evolutionary modifications.
Ventral rostrosoma, lateral lips
In Solifugae, the lateral faces of the rostrosoma firmly connect to the medial sides of the coxae of the pedipalps. At the anterior end it forms a pair of lateral lips around the mouth opening. These lateral lips are hemolymph filled and we assume that they can be moved by changing hemolymph pressure. The paired nature of the lateral lips is obvious from a thin connective tissue septum that separates left and right hemolymphatic spaces along the entire rostrosoma. The interpretation of the lateral lips as derived from endites of the pedipalps is supported by their continuous cuticular connection with the pedipalpal coxae.
The rostrosoma of Solifugae has a distinct ventromedian sclerite. This ventromedian sclerite and associated muscles are unique to solifuges (present in all four species studied; Fig. 14). The sclerite has been described as a prosternum by Börner (1902, 1904), as a deutosternum by Millot and Vachon (1949) and Snodgrass (1948), and as a tritosternum by Kästner (1931b). Van der Hammen (1989) considered it a “labium”, which, in his terminology, is the sternal part of segment II. Runge and Wirkner (2020) described it as “corresponding” to a sternum, thus remain unclear in what they precisely mean in terms of comparative evolutionary morphology. Dunlop and Lamsdell (2017) state that there was no sternum in Solifugae. Shultz (1990) encoded it as “narrow intercoxal sternal region”, a description we adopt here because it avoids a comparative anatomical assignment as a sternum. While the developmental and evolutionary origin of this sclerite remains unresolved, we note that this element is unique for Solifugae. Its topography and its association with musculature suggest that it functions as an accessory rostrosoma suction pump. Along its entire length, strong musculature spans between the sclerite and the ventrolateral wall of the rostrosoma. Depending on the assumed action of the ventral rostrosoma musculature (synchronized or consecutive), we suggest that the sclerite supports a piston-like or seesaw-like pumping mechanism of the pharynx.
Pseudoscorpions have lateral lips and an unpaired ventral lip [lophognath, Chamberlin (1931); Snodgrass (1948); “labium” in van der Hammen (1989); Fig. 14]. The lateral lips emerge from the medial face of the pedipalpal coxae and interlock ventrally with the lamina inferior. They do not contribute to the formation of the rostrosoma but built the ventral closure of a preoral space. A homologization with endites of the pedipalps has already been suggested by Kästner (1931b) and appears straightforward because of their topography and continuous connection with the coxae of the pedipalps. However, their morphology suggest that they evolved independently and parallel as compared to Solifugae.
The ventral lip of pseudoscorpions is a unique structure and has no apparent equivalent in solifuges or Acari. Chamberlin (1931; p. 97, Figs. 22, 23) suggested that the lower lip derived from the posterior margin of the mouth opening. However, it has also been homologized with a (pro)sternum (Pocock 1902), a deutosternum (Kästner 1931b, p. 733) or a tritosternum (van der Hammen 1989). Shultz (2007) pointed out that no apparent morphological features, such as borders of sclerites or muscle attachments, reliably demarcate the ventral body wall of one somite from that of an adjacent somite. However, despite this uncertainty Shultz (2007) codes the lower lip of pseudoscorpions and the lateral lips of Solifugae as “sternapophyses (= tritosternum) incorporated into the rostrosoma”. Dunlop and Lamsdell (2017) state “there is no sternum in pseudoscorpions” (p. 15). As already noted by Shultz (2007), we cannot support or reject any one of the existing interpretations. We leave it open if the lower lip of the pseudoscorpions is derived from a tritosternum, the pedipalpal coxae or is a structure that evolved independently in pseudoscorpions.
This is unfortunate because the homologization of the lower lip of pseudoscorpions with the ventromedian sclerite of the rostrosoma in Solifugae would be important to support or reject the hypothesis of the rostrosoma being a shared derived character of Haplocnemata. Our comparison of these structures shows little correspondence besides topographic similarity: the ventromedian sclerite of Solifugae is distinct as a sclerite and reaches from the tip of the rostrosoma to the apodeme between the coxae of the first walking leg; it does not merge with the pedipalpal coxae. In contrast, the lower lip of pseudoscorpions fuses with the rostrosoma and basally with the pedipalps where it forms a strong apodeme. Most conspicuous is the large muscle of Solifugae spanning between the ventromedian sclerite and the lateral wall of the rostrosoma (Fig. 14). Such muscle is missing in Pseudoscorpions, where the lower lip is free of muscles. Therefore, we suggest that the lower lip of pseudoscorpions and the ventral medial sclerite of the rostrosoma of Solifugae are structures that evolved convergently. However, even if future studies will provide evidence that both structures derive from the same morphological element, e.g., the tritosternum, their differences would be interpreted as results of independent parallel evolution of a homologous element; thus the interpretation would change from convergent to parallelism. Currently, there is no support for the idea that both sclerites were a shared autapomorphy of Solifugae and Pseudoscorpiones.
In a broader comparative framework, a tritosternum has been described for Acari (Schulze 1935; van der Hammen 1989), Palpigradi (Franz-Guess and Starck 2020), Araneae, Amblypygi, Thelyphonida, Schizomida, Ricinulei (Shultz 1990) but not for Xiphosurida. If the lower lip of pseudoscorpions and the ventromedian sclerite of the rostrosoma of Solifugae were indeed derived from a tritosternum they are homologous (symplesiomorphic), but independently modified in both groups. A tritosternum is present as a paired ventral element in Parasitiformes, thus represents an independent character state in this group. It is not involved in the formation of the mouthparts.
Endites/gnathocoxae of the pedipalps
Endites/gnathocoxae of the pedipalps occur in numerous clades of the chelicerates and are a symplesiomorphic feature of euchelicerates that is frequently associated with the ventral closure of the preoral space (Börner 1902; Kästner 1925, 1931b; Aria and Caron 2017; Haug et al. 2019; Haug 2020; Howard et al. 2020). Occurrence of endites on the pedipalps therefore must be considered a plesiomorphic euchelicerate condition that cannot be indicative of a possible relationship between Acari and Haplocnemata. Rather it appears obvious that endites/gnathocoxae of the pedipalps have been modified independently in clades of the chelicerates. We consider lateral lips of Solifugae and pseudoscorpions as results of parallel evolution of homologous morphological elements.
Homologization of the acarine hypostome with endites of the pedipalpal coxae (= lateral lips) was proposed by van der Hammen (1983) and implicitly adopted by Dunlop (2000). No developmental, morphological, or comparative evolutionary evidence supports this suggestion. An open discussion of the possible evolution of the gnathosoma in Acari can be found in Alberti and Coons (1999). Because of the unresolved homology of the hypostome of Acari, parallelism and convergent evolution of the hypostome and lateral lips appear equally probable.
We would like to highlight that Acari actually have no true rostrosoma but a gnathosoma that has diversified into a plethora of different morphologies. The preoral canal of Ixodida is a tube through which ticks suck blood and deliver saliva (Kemp and Tatchell, 1971; Sonenshine and Anderson, 2014; Vancova et al. 2020). The mouth opening is at the basis of the preoral canal, which is formed by hypostome, pedipalps and cheliceral sheath. The epistome and labrum are small and involved only in the covering of the mouth, but do not contribute to the formation of a rostrosoma (Fig. 14).
The rostrosoma of Palpigradi has occasionally been mentioned as a comparative reference. However, Franz-Guess and Starck (2020) provided compelling microscopic anatomic evidence that the rostrosoma of Palpigradi (Eukoenenia spelaea) is a simple cuticular tube that forms the upper and the lower lip at its anterior part, surrounds the preoral cavity, the mouth and the anterior part of the pharynx. The rostrosoma of Palpigradi is a structure that evolved convergently, as it involves completely different structures as compared to the rostrosoma of Solifugae, Pseudoscorpiones or the mouth parts of Acari (Kästner 1925, 1931b; Snodgrass 1948; Dunlop 2000).
Pharyngeal suction pump
In solifuges, the pharyngeal suction pump occupies the entire rostrosoma with the musculature reaching into the anterior tip. The suction pump has dorsal (pharyngeal) dilator muscles, lateral dilator muscles and circular constrictor muscles. There are no ventral dilator muscles attaching to the pharynx. However, two packages of ventral rostrosoma muscles spanning between the lateral wall of the rostrosoma and the ventromedian sclerite might support pumping movements of the pharynx act as indirect muscles.
The pharyngeal suction pump of pseudoscorpions is in the basal part of the rostrosoma and internalized into the anterior region of the prosoma. It differs markedly from that of Solifugae because it has an anterior transversal rostrosoma muscle [Musculus compressor labri, in Chamberlin (1931); Weygoldt (1965)] spanning between both sides of the rostrosoma and a posterior lateral dilator muscle, spanning between the pharynx and the lateral wall of the coxae of the pedipalps. These muscles have no equivalent in solifuges (or Acari). The constrictor muscles of the pharynx are not circular, but rather short, straight muscles spanning horizontally between the two dorsal and two ventral arms of the X-shaped pharynx, respectively.
The precerebral suction pump of Ixodes is located in the basis capituli, i.e., basal to the preoral canal. It consists of dorsal, lateral and ventral dilator muscles. Lateral dilator muscles intermingle with the constrictor muscles. The occurrence of ventral dilator muscles has no equivalent in the other taxa compared here (Fig. 14). Also, the pharyngeal valve with its complex morphology is a unique structure of the anterior pharynx of ixodid ticks. The functional morphology of feeding and the action of the pharyngeal valve have recently been modelled (Vancova et al. 2020). Balashov (1983) documents the same set of pharyngeal muscles for Hyalomma asiaticum. Based on their topographic arrangement, dilator muscles and circular muscles act as antagonists driving peristaltic sucking movements of the pharynx. Surprisingly, ventral dilator muscles and constrictor muscles have not been considered in the functional model by Vancová et al. (2020).
Conclusions
It becomes evident from our comparisons that the mouthparts of all three clades are probably derived from a common euchelicerate ground pattern. Some elements like the epistome, the labrum and the endites of the pedipalps may represent symplesiomorphies of the arthropod ground pattern. Our detailed microscopic anatomical analysis shows that in each group, Solifugae, Pseudoscorpiones and at least the ticks within the Parasitiformes, the mouthparts possess unique features indicating independent parallel evolution of homologous morphological elements. Some elements like the ventromedial sclerite in Solifugae and the ventral lip in Pseudoscorpiones are interpreted as convergently evolved ventral closure of the rostrosoma.
We have provided evidence that the labrum is reduced in Solifugae. The entire dorsal cover of the rostrosoma is formed by an elongated epistome. This observation rejects the suggestion that an epistomo-labral plate is a synapomorphy of solifuges, pseudoscorpions and Acari. The existence of an epistome and a labrum is a symplesiomorphy of arthropods; Solifugae thus representing a derived autapomorphic reduction of the labrum.
Lateral lips derive from the medial faces of the pedipalpal coxae. However, we highlight that the lateral lips in Solifugae close the mouth ventro-laterally and contribute to the rostrosoma. In Pseudoscorpiones the lateral lips close the perioral cavity, but are not involved in the formation of the rostrosoma and the mouth. Their specific interlocking-mechanism and their asymmetry makes them highly derived. We do not attempt to solve whether the hypostome of Parasitiformes is homologous with endites of the pedipalpal coxae. If the hypostome derives from coxal endites, then it is certainly independent from those in pseudoscorpions and Solifugae and, of course, from that in other Parasitiformes. Thus, we concur with Dunlop (2000) that lateral lips are present in all three clades, but their morphology is independently derived and diverging from a symplesiomorphic character of the chelicerate ground pattern.
The dorsal pharyngeal dilator muscle is a common theme of the pharyngeal suction pump in all chelicerates. It is also present in all three taxa compared here in detail. However, the pharyngeal suction pump differs in almost all other aspects of the microscopic anatomy that we conclude that it evolved independently from that ground pattern in all three clades.
The rostrosoma of Solifuges, Pseudoscorpiones and Acari differs in so many morphological details that we conclude it evolved through independent parallel evolution of homologous elements. This view is supported by subtle differences in the design of the rostrosoma. In consequence, the rostrosoma is not suitable as a character in a morphology-based phylogeny of chelicerates; specifically, the rostrosoma of Pseudoscorpiones and Solifugae does not support Hapolocnemata.
References
Alberti G, Coons B (1999) Acari: Mites. In: Harrison FW, Foelix RF (eds) Microscopic anatomy of invertebrates, vol 8C. Wiley-Liss, New York, pp 515–1265
Alberti G, Dabert J (2012) Fine structure of the feather mite Falculifer rostratus (Buchholz, 1869) (Astigmata, Flaculiferidae). Part 1: gnathosoma with remarks on the sensory system. Zoologica 158:3–75
Alberti G, Heethoff M, Norton RA, Schmelzle S, Seniczak A, Seniczak S (2011) Fine structure of the gnathosoma of Archegozetes longisetus Aoki (Acari: Oribatida Trhypochthoniidae). J Morphol 272(9):1025–1079
Aria C, Caron JB (2017) Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evol Biol 17(1):1–20
Ax P (2003) Multicellular animals. Order in nature—system made by man (Vol III). Springer, Berlin, Heidelberg
Balashov YS (1983) An Atlas of ixodid tick ultrastructure. English translation by Raikhel AS. English publication edited by Raikhel AS and Hoogstraal H. Special Publication of the entomological Society of America. College Park, MD
Beier M (1931) Ordnung der Arachnida: Pseudoscorpionidea=Afterscorpione. Arthropoda ex Insecta. 2. Hälfte: Arthropoda: Chelicerata. In: Handbuch der Zoologie. Eine Naturgeschichte der Stämme des Tierreichs. Herausgeber, Thilo Krumbach, deGruyter
Bitsch J, Bitsch C (2007) The segmental organization of the head region in Chelicerata: a critical review of recent studies and hypotheses. Acta Zool 88(4):317–335
Börner C (1902) Arachnologische studien (II u III). Zool Anz 25:433–466
Börner C (1904) Beiträge zur Morphologie der Arthropoden: I. Ein Beitrag zur Kenntnis der Pedipalpen. Zoologica 17(42):1–174
Chamberlin JC (1931) The arachnid order chelonethida. Stanf Univ Publ Biol Sci 7:1–292
Coons LB, Alberti G (1999) Acari: ticks. In: Harrison FW, Foelix RF (eds) Microscopic anatomy of invertebrates, vol 8B. Wiley-Liss, New York, pp 267–514
Di Palma A, Alberti G, Nuzzaci G, Krantz GW (2006) Fine structure and functional morphology of the mouthparts of a male Veigaia sp. (Gamasida: Veigaiidae) with remarks on the spermatodactyl and related sensory structures. J Morphol 267(2):208–220
Di Palma A, Nuzzaci G, Alberti G (2009) Morphological ultrastructural and functional adaptations of the mouthparts in cheyletid mites (Acari: Actinedida: Cheyletidae). Int J Acarology 35:521–532
Dunlop JA (2000) The epistomo-labral plate and lateral lips in solifuges pseudoscorpions and mites. Ekologia (bratislava) 19(Suppl 3):67–78
Dunlop JA, Lamsdell JC (2017) Segmentation and tagmosis in Chelicerata. Arthropod Struct Dev 46(3):395–418
Farley RD (1999) Ventral mesosomal changes in embryos from three scorpion families. In: Iuridae, Buthidae and Vaejovidae (eds) Proceedings of the XIV international congress of arachnology and a symposium on spiders in agroecosystems. J Arachnology 27(1):123–128
Felgenhauer BE (1999) Araneae. In: Harrison FW, Foelix RF (eds) Microscopic anatomy of invertebrates, vol 8A. Wiley-Liss, New York, pp 223–266
Filimonova SA, Mironov SV (2010) Functional morphology of the gnathosoma in the quill mite Syringophilopsis fringilla Fritsch (Acari: Prostigmata: Syringophilidae). Zool Anz 249(3–4):165–180
Franz-Guess S, Starck JM (2020) Microscopic anatomy of Eukoenenia spelaea (Peyerimhoff 1902) (Arachnida:Palpigradi: Eukoeneniidae). Bonn Zool Bull Suppl 65:1–125
Franz-Guess S, Klußmann-Fricke BJ, Wirkner CS, Prendini L, Starck JM (2016) Morphology of the tracheal system of camel spiders (Chelicerata: Solifugae) based on micro-CT and 3D-reconstruction in exemplar species from three families. Arthropod Struct Dev 45(5):440–451
Giribet G (2018) Current views on chelicerate phylogeny—a tribute to Peter Weygoldt. Zool Anz 273:7–13
Giribet G, Edgecombe GD, Wheeler WC, Babbitt C (2002) Phylogeny and systematic position of Opiliones: a combined analysis of chelicerate relationship using morphological and molecular data. Cladistics 18:5–70
Hall BK (2007) Homoplasy and homology: dichotomy or continuum? J Hum Evol 52(5):473–479
Haug C (2020) The evolution of feeding within Euchelicerata: data from the fossil groups Eurypterida and Trigonotarbida illustrate possible evolutionary pathways. PeerJ 8:e9696
Haug C, Rötzer MA (2018) The ontogeny of the 300 million year old xiphosuran Euproops danae (Euchelicerata) and implications for resolving the Euproops species complex. Dev Genes Evol 228(1):63–74
Haug C, Wagner P, Haug JT (2019) The evolutionary history of body organisation in the lineage towards modern scorpions. Bull Geosci 94(4):389–408
Howard RJ, Puttick MN, Edgecombe GD, Lozano-Fernandez J (2020) Arachnid monophyly: morphological palaeontological and molecular support for a single terrestrialization within Chelicerata. Arthropod Struct Dev. https://doi.org/10.1016/j.asd.2020.100997
Kästner A (1925) Vergleichend-morphologische Untersuchungen der Gnathocoxen der Araneen. Z Morphol Okol Tiere 4(5):711–738
Kästner A (1931a) Die Hüfte und ihre Umformung zu Mundwerkzeugen bei den Arachniden. Z Morphol Okol Tiere 22(2–3):721–758
Kästner A (1931b) Ordnung solifugae (Walzenspinnen). In: Krumbach T (ed) Handbook of zoology online, vol 2. De Gruyter, Berlin/Boston, pp 193–299
Kemp DH, Tatchell RJ (1971) The mechanism of feeding and salivation in Boophilus microplus (Canestrini 1887). Z Parasitenkd 37(1):55–69
Kimm MA, Prpic NM (2006) Formation of the arthropod labrum by fusion of paired and rotated limb-bud-like primordia. Zoomorphology 125(3):147–155
Klann AE (2009) Histology and ultrastructure of Solifugae—Comparative studies of organ systems of solifuges (Arachnida Solifugae) with special focus on functional analyses and phylogenetic interpretations. Dissertation, University of Greifswald
Klann AE, Alberti G (2010) Histological and ultrastructural characterization of the alimentary system of solifuges (Arachnida Solifugae). J Morphol 271(2):225–243
Lozano-Fernandez J, Tanner AR, Giacomelli M, Carton R, Vinther J, Edgecombe GD, Pisani D (2019) Increasing species sampling in chelicerate genomic-scale datasets provides support for monophyly of Acari and Arachnida. Nat Commun 10:2295. https://doi.org/10.1038/s41467-019-10244-7
Ludwig M, Palacios-Vargas JG, Alberti G (1994) Cellular details of the midgut of Cryptocellus boneti (Arachnida: Ricinulei). J Morphol 220:263–270
Mehnert L, Dietze Y, Hörnig MK, Starck JM (2018) Body tagmatization in pseudoscorpions. Zool Anz 273:152–163
Millot J, Vachon M (1949) Ordre des Solifuges. Traité de Zoologie Anatomie Systématique Biologie. Tome VI. Masson et Cie, Paris, pp 487–519
Pinto-da-Rocha R, Machado G, Giribet G (2007) Harvestmen. The biology of opiliones. Harvard University Press, Cambridge/London
Pocock RI (1902) Studies on the arachnid endosternite. Q J Microsc Sci 46:225–262 (plates 13–14)
Rüdeberg C (1967) A rapid method for staining thin sections of vestopal W-embedded tissue for light microscopy. Experientia 23:792–792
Runge J, Wirkner CS (2020) Evolutionary and functional substitution of extrinsic musculature in Solifugae (Arachnida). J Morphol 281(12):1524–1533
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological developmental and paleontological evidence. Dev Genes Evol 216(7–8):395–415
Schulze P (1935) Zur vergleichenden Anatomie der Zecken. (Das Sternale, die Mundwerkzeuge, Analfurchen und Analbeschilderung, ihre Bedeutung, Ursprünglichkeit und Luxurieren). Z Morphol Okol Tiere 30:1–40
Sharma PP, Kaluziak ST, Pérez-Porro AR, González VL, Hormiga G, Wheeler WC, Giribet G (2014) Phylogenomic interrogation of Arachnida reveals systemic conflicts in phylogenetic signal. Mol Biol Evol 31(11):2963–2984
Shatrov AB (2011) Comparative and functional morphology of the mouthparts in larvae of Parasitengona (Acariformes). Zoosymposia 6(1):14–23
Shatrov AB (2012) Functional morphology and ultrastructure of mouthparts in unfed water mite larvae Piona carnea (Koch, 1836) (Acariformes: Pionidae). Zool Anz 251(2):85–100
Shultz JW (1990) Evolutionary morphology and phylogeny of Arachnida. Cladistics 6(1):1–38
Shultz JW (2007) A phylogenetic analysis of the arachnid orders based on morphological characters. Zool J Linn Soc 150:221–265
Snodgrass RE (1948) The feeding organs of Arachnida including mites and ticks. Smithson Misc Collect 110:1–93
Sonenshine DE, Anderson JM (2014) Mouthparts and digestive system: anatomy and molecular biology of feeding and digestion. In: Sonenshine DE, Roe MR (eds) Biology of ticks, vol 1, 2nd edn. Oxford University Press, New York, pp 74–98
Starck JM, Mehnert L, Biging A, Bjarsch J, Franz-Guess S, Kleeberger D, Hörnig M (2018) Morphological responses to feeding in ticks (Ixodes ricinus). Zool Lett 4(1):1–19
Talarico G, Lipke E, Alberti G (2011) Gross morphology histology and ultrastructure of the alimentary system of Ricinulei (Arachnida) with emphasis on functional and phylogenetic implications. J Morphol 272:89–117
Vancová M, Bílý T, Šimo L, Touš J, Horodyský P, Růžek D, Novobilský A, Salát J, Strnad M, Sonenshine DE, Grubhoffer L, Nebesářová J (2020) Three-dimensional reconstruction of the feeding apparatus of the tick Ixodes ricinus (Acari: Ixodidae): a new insight into the mechanism of blood-feeding. Sci Rep 10(1):1–7
van der Hammen L (1977) A new classification of Chelicerata. Zool Meded 51:307–319
van der Hammen L (1983) Notes on the comparative morphology of ticks (Anactinotrichida: Ixodida). Zool Meded 57:209–242
van der Hammen L (1986a) Comparative studies in Chelicerata IV. Apatellata Arachnida Scorpionida Xiphosura. Zool Verh 226(1):1–52
van der Hammen L (1986b) Acarological and arachnological notes. Zool Meded 60(14):217–230
van der Hammen L (1989) An introduction to comparative arachnology. SPB Academic Publishing, Leiden
Wake DB, Wake MH, Specht CD (2011) Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science 331(6020):1032–1035
Weygoldt P (1964) Vergleichend embryologische Untersuchungen an Pseudoscorpionen (Chelonethi). Z Morphol Oekol Tiere 54:1–106
Weygoldt P (1965) Vergleichend-embryologische Untersuchungen an Pseudoscorpionen III. Z Morphol Okol Tiere 55(3):321–382
Weygoldt P (1968) Vergleichend-embryologische Untersuchungen an Pseudoscorpionen. Zeitschrift Für Morphologie Der Tiere 63(2):111–154
Weygoldt P (1969) The biology of pseudoscorpions (No 6). Harvard University Press, Cambridge
Weygoldt P (1971) Vergleichend-embryologische Untersuchungen an Pseudoscorpionen V. Das Embryonalstadium mit seinem Pumporgan bei verschiedenen Arten und sein Wert als taxonomisches Merkmal. J Zool Syst Evol Res 9(1):3–29
Weygoldt P (1998) Evolution and systematics of the Chelicerata. Exp Appl Acarol 22(2):63–79
Weygoldt P, Paulus HF (1979) Untersuchungen zur Morphologie Taxonomie und Phylogenie der Chelicerata 1 II. Cladogramme und die Entfaltung der Chelicerata. Zeitschrift Für Zoologische Systematik Und Evolutionsforschung 17(3):177–200
Acknowledgements
We thank Anja Klann and Peter Michalik, University of Greifswald, for granting access to the histological material of Solifugae. We thank Antoinette von Sigriz-Pesch, Sabine Sass, and Christine Dunkel for technical assistance with all histology work (Pseudoscorpiones, Ixodes).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JMS: Conceptualization (lead); supervision; investigation (equal); formal analysis (equal); writing—original draft (lead); formal analysis (lead); visualization (lead); writing—review and editing (lead). JB: investigation (Solifugae µCT); formal analysis (equal); visualization (µCT-model Solifugae); writing—review and editing (supporting). JB: investigation (Solifugae histology); formal analysis (Solifugae rostrosoma) writing –review and editing (supporting). LM: investigation (Pseudoscorpiones histology and µCT); formal analysis (Pseudoscorpiones rostrosoma); writing—review and editing (supporting).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. No funding was received for conducting this study.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because the work was exclusively conducted with collection material.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starck, J.M., Belojević, J., Brozio, J. et al. Comparative anatomy of the rostrosoma of Solifugae, Pseudoscorpiones and Acari. Zoomorphology 141, 57–80 (2022). https://doi.org/10.1007/s00435-021-00551-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-021-00551-3