Abstract
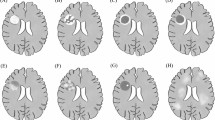
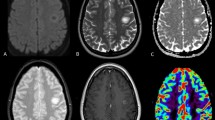
Tumefactive demyelinating lesions (TDLs) can mimic brain tumors on radiological images. TDLs are often referred to as tumefactive multiple sclerosis (TMS), but the heterogeneous nature and monophasic course of TDLs do not fulfill clinical and magnetic resonance imaging (MRI) criteria for multiple sclerosis. Redefining TDLs, TMS and other inflammatory brain lesions is essential for the accurate clinical diagnosis of extensive demyelinating brain lesions. We retrospectively analyzed MRI from nine TDL cases that underwent brain biopsy. Patterns of gadolinium enhancement on MRI were categorized as homogenous, inhomogeneous, patchy and diffuse, open ring or irregular rim, and were compared with pathological hallmarks including demyelination, central necrosis, macrophage infiltration, angiogenesis and perivascular lymphocytic cuffing. All cases had coexistence of demyelinating features and axonal loss. Open-ring and irregular rim patterns of gadolinium enhancement were associated with macrophage infiltrations and angiogenesis at the inflammatory border. An inhomogeneous pattern of gadolinium enhancement was associated with perivascular lymphocytic cuffing. Central necrosis was seen in cases of severe multiple sclerosis and hemorrhagic leukoencephalopathy. These results suggest that the radiological features of TDLs may be related to different pathological processes, and indicate that MRI may be useful in understanding their pathophysiology. Further investigation is needed to determine the precise disease entity of these inflammatory demyelinating brain lesions.


Similar content being viewed by others
References
Lucchinetti CF, Gavrilova RH, Metz I et al (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 131:1759–1775
Siva A (2006) The spectrum of multiple sclerosis and treatment decisions. Clin Neurol Neurosurg 108:333–338
Dagher AP, Smirniotopoulos J (1996) Tumefactive demyelinating lesions. Neuroradiology 38:560–565
Kiriyama T, Kataoka H, Taoka T et al (2011) Characteristic neuroimaging in patients with tumefactive demyelinating lesions exceeding 30 mm. J Neuroimaging 21:e69–e77
Brück W, Bitsch A, Kolenda H et al (1997) Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 42:783–793
Nesbit GM, Forbes GS, Scheithauer BW et al (1991) Multiple sclerosis: histopathological and MR and/or CT correlation in 37 cases at biopsy and three cases at autopsy. Radiology 180:467–474
Takahashi T, Fujihara K, Nakashima I et al (2006) Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med 210:307–313
Noseworthy JH, Lucchinetti C, Rodrequez M et al (2000) Multiple sclerosis. N Engl J Med 343:938–952
Wingerchuk DM, Lennon VA, Lucchinetti CF et al (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6:805–815
Wingerchuk DM, Hogancamp WF, O’Brien PC et al (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53:1107–1114
Tintore M, Rovira A, Rio J et al (2003) New diagnostic criteria for multiple sclerosis: application in first demyelinating episode. Neurology 60:27–30
Van Waesberghe JH, Kamphorst W, De Groot CJ et al (1999) Axonal loss in MS lesions: MR insight into substrate of disability. Ann Neurol 46:1289–1293
Given CA 2nd, Stevens CS, Lee C (2004) The MRI appearance of tumefactive demyelinating lesions. Am J Radiol 182:195–199
Walner-Blazek M, Rovira A, Filippi M et al (2013) Atypical idiopathic inflammatory demyelinating lesions: prognostic implications and relation to multiple sclerosis. J Neurol 260:2016–2022
Rossi A (2008) Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am 18:149–161
Young NP, Weinshenker BG, Parisi JE et al (2010) Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain 133:333–348
Gibbs WN, Kreidie MA, Kim RC et al (2005) Acute hemorrhagic leukoencephalitis neuroimaging features and neuropathologic diagnosis. J Comput Assist Tomogr 29:689–693
Xia L, Lin S, Wang ZC et al (2009) Tumefactive demyelinating lesions: nine cases and a review of the literature. Neurosurg Rev 32:171–179
Morrissey SP, Stodal H, Zettl U et al (1996) In vivo MRI and its histological correlations in acute adoptive transfer experimental allergic encephalomyelitis. Quantification of inflammation and edema. Brain 119:239–248
Schwartz KM, Erickson BJ, Lucchinetti CF (2006) Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology 48:143–149
Kirk SL, Karlik SJ (2003) VEGF and vascular changes in chronic neuroinflammation. J Autoimmune 21:353–363
Su JJ, Osoegawa M, Matsuoka T et al (2006) Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. J Neurol Sci 243:21–30
Proesholdt MA, Jacobson S, Tresser N et al (2002) Vascular endothelial growth factor is expressed in multiple sclerosis plaque and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol 61:914–925
Sorensen TL, Ransohoff RM, Strieter RM et al (2004) Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol 11:445–449
Kobayashi M, Ono Y, Shibata N et al (2009) Correlation between magnetic resonance imaging findings and pathological observations in tumefactive multiple sclerosis. Neuroradiol J 22:155–163
Acknowledgments
The authors would like to thank Drs. T. Maruyama, Y. Muragaki, T. Ochiai, and O. Kubo for biopsy procedures and valuable suggestions. The authors thank Dr. Takahashi (Department of Neurology, Tohoku University) for the analysis of AQP-4 antibodies. A summary of this paper was reported at the 61st meeting of the American Academy of Neurology in 2009. This study was supported by a Health and Labour Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflict of interest.
Ethical standards
The ethics committee at Tokyo Women’s Medical University approved this study. Owing to the retrospective nature of the study, ethics approval was not mandatory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, M., Shimizu, Y., Shibata, N. et al. Gadolinium enhancement patterns of tumefactive demyelinating lesions: correlations with brain biopsy findings and pathophysiology. J Neurol 261, 1902–1910 (2014). https://doi.org/10.1007/s00415-014-7437-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7437-1