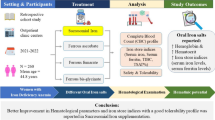
Abstract
Purpose
Iron deficiency anaemia (IDA) is a global public health problem. Treatment with the standard of care ferrous iron salts may be poorly tolerated, leading to non-compliance and ineffective correction of IDA. Employing supplements with higher bioavailability might permit lower doses of iron to be used with fewer side effects, thus improving treatment efficacy. Here, we compared the iron bioavailability of ferrous sulphate tablets with alternative commercial iron products, including three liquid-based supplements.
Methods
Iron bioavailability was measured using Caco-2 cells with ferritin formation as a surrogate marker for iron uptake. Statistical analysis was performed using one-way ANOVA followed by either Dunnett’s or Tukey’s multiple comparisons tests.
Results
Spatone Apple® (a naturally iron-rich mineral water with added ascorbate) and Iron Vital F® (a synthetic liquid iron supplement) had the highest iron bioavailability. There was no statistical difference between iron uptake from ferrous sulphate tablets, Spatone® (naturally iron-rich mineral water alone) and Pregnacare Original® (a multimineral/multivitamin tablet).
Conclusion
In our in vitro model, naturally iron-rich mineral waters and synthetic liquid iron formulations have equivalent or better bioavailability compared with ferrous iron sulphate tablets. If these results are confirmed in vivo, this would mean that at-risk groups of IDA could be offered a greater choice of more bioavailable and potentially better tolerated iron preparations.


Similar content being viewed by others
References
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B (2009) Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr 12(4):444–454. doi:10.1017/S1368980008002401
Shankar P, Boylan M, Sriram K (2010) Micronutrient deficiencies after bariatric surgery. Nutrition 26(11–12):1031–1037. doi:10.1016/j.nut.2009.12.003
Miller JL (2013) Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect. doi:10.1101/cshperspect.a011866
Andrews NC (1999) Disorders of iron metabolism. New Engl J Med 341(26):1986–1995. doi:10.1056/NEJM199912233412607
Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C, British Committee for Standards in H (2012) UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 156(5):588–600
Zhou SJ, Gibson RA, Crowther CA, Makrides M (2009) Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose-response trial. Eur J Clin Nutr 63(2):183–190. doi:10.1038/sj.ejcn.1602926
Hyder SM, Persson LA, Chowdhury AM, Ekstrom EC (2002) Do side-effects reduce compliance to iron supplementation? A study of daily- and weekly-dose regimens in pregnancy. J Health Popul Nutr 20(2):175–179
Seck BC, Jackson RT (2008) Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutr 11(6):596–605. doi:10.1017/S1368980007000924
Habib F, Alabdin EH, Alenazy M, Nooh R (2009) Compliance to iron supplementation during pregnancy. J Obstet Gynaecol 29(6):487–492. doi:10.1080/01443610902984961
Keller J, Frederking D, Layer P (2008) The spectrum and treatment of gastrointestinal disorders during pregnancy. Nat Clin Pract Gastr 5(8):430–443. doi:10.1038/ncpgasthep1197
Bonapace ES Jr, Fisher RS (1998) Constipation and diarrhea in pregnancy. Gastroenterol Clin N 27(1):197–211
Beard JL (2000) Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr 71(5 Suppl):1288S–1294S
Ekstrom EC, Kavishe FP, Habicht JP, Frongillo EA Jr, Rasmussen KM, Hemed L (1996) Adherence to iron supplementation during pregnancy in Tanzania: determinants and hematologic consequences. Am J Clin Nutr 64(3):368–374
Stoltzfus RJ (2011) Iron interventions for women and children in low-income countries. J Nutr 141(4):756S–762S. doi:10.3945/jn.110.128793
Worwood M, Evans WD, Villis RJ, Burnett AK (1996) Iron absorption from a natural mineral water (Spatone Iron-Plus). Clin Lab Haematol 18(1):23–27
Halksworth G, Moseley L, Carter K, Worwood M (2003) Iron absorption from Spatone (a natural mineral water) for prevention of iron deficiency in pregnancy. Clin Lab Haematol 25(4):227–231
Zariwala MG, Somavarapu S, Farnaud S, Renshaw D (2013) Comparison study of oral iron preparations using a human intestinal model. Sci Pharm 81(4):1123–1139. doi:10.3797/scipharm.1304-03
Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD (1998) Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr 128(9):1555–1561
Yun S, Habicht JP, Miller DD, Glahn RP (2004) An in vitro digestion/Caco-2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. J Nutr 134(10):2717–2721
Caro IBX, Rousset M, Meunier V, Bourrie M, Julian B, Joyeux H, Roques C, Berger Y, Zweibaum A, Fabre G (1995) Characterisation of a newly isolated Caco-2 clone (TC-7), as a model of transport processes and biotransformation of drugs. Int J Pharm 116:147–158
Sharp P, Tandy S, Yamaji S, Tennant J, Williams M, Singh Srai SK (2002) Rapid regulation of divalent metal transporter (DMT1) protein but not mRNA expression by non-haem iron in human intestinal Caco-2 cells. FEBS Lett 510(1–2):71–76
Christides T, Sharp P (2013) Sugars increase non-heme iron bioavailability in human epithelial intestinal and liver cells. PLoS One 8(12):e83031. doi:10.1371/journal.pone.0083031
Glahn RP, Rassier M, Goldman MI, Lee OA, Cha J (2000) A comparison of iron availability from commercial iron preparations using an in vitro digestion/Caco-2 cell culture model. J Nutr Biochem 11(2):62–68
Motulsky H (2010) Intuitive biostatistics, 2nd edn. Oxford University Press, Oxford
McKenna D, Spence D, Haggan SE, McCrum E, Dornan JC, Lappin TR (2003) A randomized trial investigating an iron-rich natural mineral water as a prophylaxis against iron deficiency in pregnancy. Clin Lab Haematol 25(2):99–103
Teucher B, Olivares M, Cori H (2004) Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res 74(6):403–419
Olivares M, Pizarro F, Ruz M, de Romana DL (2012) Acute inhibition of iron bioavailability by zinc: studies in humans. Biometals 25(4):657–664. doi:10.1007/s10534-012-9524-z
Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81(5):1218S–1222S
CDC (1998) Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep, vol 47. Centers for Disease Control and Prevention
WHO (World Health Oreganization) (2012) Guideline: daily iron and folic acid supplementation in pregnant women. Geneva, World Health Organization
WHO (World Health Organization) (2012) Guideline: intermittent iron and folic acid supplementation in non-anaemic pregnant women. Geneva, World Health Organization
NICE (2008) Antenatal care: routine care for the healthy pregnant woman. National Institute for Clinical Excellence, London
Fenton V, Cavill I, Fisher J (1977) Iron stores in pregnancy. Br J Haematol 37(1):145–149
Cuervo LG, Mahomed K (2001) Treatments for iron deficiency anaemia in pregnancy. Cochrane Database Syst Rev. doi:10.1002/14651858.CD003094
Pena-Rosas JP, Viteri FE (2009) Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004736.pub3
Krafft A (2013) Iron supplementation in pregnancy. Br Med J 347:f4399. doi:10.1136/bmj.f4399
Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE (2012) Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004736.pub4
Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW, Nutrition Impact Model Study G (2013) Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. Br Med J 346:f3443. doi:10.1136/bmj.f3443
Jauregui-Lobera I (2013) Iron deficiency and bariatric surgery. Nutrients 5(5):1595–1608. doi:10.3390/nu5051595
Bal BS, Finelli FC, Shope TR, Koch TR (2012) Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol 8(9):544–556. doi:10.1038/nrendo.2012.48
Stein J, Stier C, Raab H, Weiner R (2014) Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharm Ther 40(6):582–609. doi:10.1111/apt.12872
Sawaya RA, Jaffe J, Friedenberg L, Friedenberg FK (2012) Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab 13(9):1345–1355
Gasteyger C, Suter M, Gaillard RC, Giusti V (2008) Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr 87(5):1128–1133
Gesquiere I, Lannoo M, Augustijns P, Matthys C, Van der Schueren B, Foulon V (2014) Iron deficiency after Roux-en-Y gastric bypass: insufficient iron absorption from oral iron supplements. Obes Surg 24(1):56–61. doi:10.1007/s11695-013-1042-8
Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, Vickers SM (2006) Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg 72(12):1196–1202
Netto BD, Moreira EA, Patino JS, Beninca JP, Jordao AA, Frode TS (2012) Influence of Roux-en-Y gastric bypass surgery on vitamin C, myeloperoxidase, and oral clinical manifestations: a 2-year follow-up study. Nutr Clin Pract 27(1):114–121. doi:10.1177/0884533611431462
Fairweather-Tait S, Lynch S, Hotz C, Hurrell R, Abrahamse L, Beebe S, Bering S, Bukhave K, Glahn R, Hambidge M, Hunt J, Lonnerdal B, Miller D, Mohktar N, Nestel P, Reddy M, Sandber AS, Sharp P, Teucher B, Trinidad TP (2005) The usefulness of in vitro models to predict the bioavailability of iron and zinc: a consensus statement from the HarvestPlus expert consultation. Int J Vitam Nutr Res 75(6):371–374
Acknowledgments
This work was supported by the Faculty of Engineering and Science at the University of Greenwich, and the Diabetes and Nutritional Sciences Division at King’s College London. We thank David Scott Ganis for assistance with statistical and numerical analysis.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Christides, T., Wray, D., McBride, R. et al. Iron bioavailability from commercially available iron supplements. Eur J Nutr 54, 1345–1352 (2015). https://doi.org/10.1007/s00394-014-0815-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0815-8