Abstract
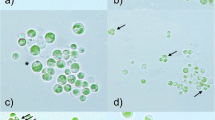
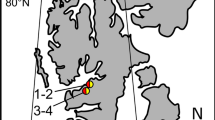
At the arctic archipelago of Svalbard, bare glacier surfaces are populated by microalgae like Ancylonema nordenskiöldii (Zygnematales, Streptophyta). The resulting blooms cause, due to a vacuolar pigmentation, brownish colourations of the glacier surface. This freshwater ice alga has been described from several polar and alpine glaciers; however, these reports lacked data about the ecophysiology or ultrastructure. Considering the harsh environmental conditions of the exceptional habitat, such as permanently low temperatures, exposure to high irradiation or a short vegetation period, the aim of this study was to elucidate cellular adaptations of A. nordenskiöldii. Thus, samples were collected at two glaciers in Spitsbergen. The cytoarchitecture of the cylindrical cells, which are arranged in unbranched filaments, demonstrates active cells with Golgi bodies, mitochondria and rough endoplasmic reticulum close to the nucleus when investigated by transmission electron microscopy (TEM). The cell walls are pore less and only 90 nm thin. A. nordenskiöldii only sporadically produces oblong zygotes when two filaments conjugate. The most remarkable cytological feature is peripheral brownish vacuoles, appearing osmiophil and electron dense by TEM. Aqueous extracts of this pigmentation show a broad absorption in the visible light and in the UV. Consequently, a protection against excessive irradiation is provided. Photosynthesis measurements performed at different temperatures and light levels indicate that the metabolism is adapted to temperatures close to the freezing point as well as to high light conditions. Therefore, A. nordenskiöldii can be regarded as metabolically and cytological well adapted to live on glaciers.





Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- VIS:
-
Visible light
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet light
References
Chodat R (1896) La Flore des Neiges du Col des Ecandies. Bull Herb Boiss 4:879–889
Gontcharov AA, Marin B, Melkonian M (2003) Molecular phylogeny of conjugating green algae (Zygnematophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. J Mol Evol 56:89–104
Hadacek F, Bachmann G, Engelmeier D, Chobot V (2011) Hormesis and a chemical raison d’être for secondary plant metabolites. Dose-Response 9:79–116
Hanelt D, Tüg H, Bischof K, Gross C, Lippert H, Sawall T, Wiencke C (2001) Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth. Mar Biol 138:649–658
Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Hoham RW, Duval B (2001) Microbial ecology of snow and freshwater ice with emphasis on snow algae. In: Jones HG, Pomeroy JW, Walker DA, Hoham RW (eds) Snow ecology. Cambridge University Press, New York, pp 168–228
Hoham RW, Filbin RW, Frey FM, Pusack TJ, Ryba JB, McDermott PD, Fields RA (2007) The optimum pH of the green snow algae, Chloromonas tughillensis and Chloromonas chenangoensis, from Upstate New York. Arct Antarct Alp Res 39:65–73
Holzinger A, Lütz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207
Holzinger A, Karsten U, Lütz C, Wiencke C (2006) Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa (Lightfoot) Kützing from Spitsbergen (Norway) under UV exposure. Phycologia 45:168–177
Holzinger A, Roleda MY, Lütz C (2009) The vegetative arctic green alga Zygnema is insensitive to experimental UV exposure. Micron 40:831–838
Holzinger A, Tschaikner A, Remias D (2010) Cytoarchitecture of the desiccation-tolerant green alga Zygogonium ericetorum. Protoplasma 243:15–24
Holzinger A, Lütz C, Karsten U (2011) Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. J Phycol 47:591–602
Karsten U, Holzinger A (2011) Light, temperature and desiccation effects on photosynthetic activity and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microb Ecol. doi:10.1007/s00248-011-9924-6
Kaštovská K, Stibal M, Šabacká M, Černá B, Elster J (2007) Microbial community structure and ecology of subglacial sediments in two polythermal Svalbard glaciers characterized by epifluorescence microscopy and PLFA. Polar Biol 30:277–287
Kim GH, Klochkova TA, Han WH, Kang SH, Choi HG, Chung KIW, Song JK (2011) Freshwater and terrestrial algae from Ny-Alesund and Blomstrandhalvoya Island (Svalbard). Arctic 64:25–31
Kol E (1968) Kryobiologie. Biologie und Limnologie des Schnees und Eises. I. Kryovegetation. Die Binnengewässer, Band XXIV. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Kol E, Eurola S (1974) Red snow algae from Spitsbergen. Astarte 7:61–66 (J Arct Biol)
Komárek O, Komárek J (2001) Contribution to the taxonomy and ecology of cryosestic algae in the summer season 1995–96 at King George Island, S. Shetland Islands. Beih Nova Hedwigia 123:121–140
Komárek J, Nedbalová L (2008) Green cryosestic algae. In: Seckbach J (ed) Cellular origin, life in extreme habitats and astrobiology (volume 11): algae and cyanobacteria in extreme environments, part 4: phototrophs in cold environments. Springer, Dordrecht, pp 323–344
Leya T (2004) Feldstudien und genetische Untersuchungen zur Kryophilie der Schneealgen Nordwestspitzbergens. Dissertation. Shaker, Aachen
Ling HU, Seppelt RD (1990) Snow algae of the Windmill Islands, continental Antarctica. Mesotaenium berggrenii (Zygnematales, Chlorophyta) the alga of grey snow. Antarct Sci 2:143–148
Margesin R, Schinner F (1999) Cold-adapted organisms. Ecology, physiology, enzymology and molecular biology. Springer, Berlin
Matuła J, Pietryka M, Richter D, Wojtuń B (2007) Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area, Spitsbergen. Polish Polar Res 28:283–315
McCourt RM, Karol KG, Bell J, Helm-Bychowski KM, Grajewska A, Wojciechowski MF, Hoshaw RW (2000) Phylogeny of the conjugating green algae (Zygnemophyceae) based on rbcL sequences. J Phycol 36:747–758
Mix M (1972) Die Feinstruktur der Zellwände bei Mesotaeniaceae und Gonatophyceae mit einer vergleichenden Betrachtung der verschiedenen Wandtypen der Conjugatophyceae und über deren systematischen Wert. Arch Mikrobiol 81:197–220
Mueller DR, Pollard WH (2004) Gradient analysis of cryoconite ecosystems from two polar glaciers. Polar Biol 27:66–74
Remias D (2012) Cell structure and physiology of alpine snow and ice algae. In: Lütz C (ed) Plants in alpine regions. Cell physiology of adaption and survival strategies. Springer, Wien, pp 175–186
Remias D, Holzinger A, Lütz C (2009) Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia 48:302–312
Remias D, Albert A, Lütz C (2010) Effects of realistically simulated, elevated UV irradiation of photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the arctic soil alga Tetracystis sp. (Chlorophyceae). Photosynthetica 48:302–312
Remias D, Schwaiger S, Aigner S, Leya T, Stupper H, Lütz C (2011) Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol Ecol. doi:10.1111/j.1574-6941.2011.01245.x
Stibal M, Elster J, Šabacká M, Kaštovská K (2007) Seasonal and diel changes in photosynthetic activity of the snow alga Chlamydomonas nivalis (Chlorophyceae) from Svalbard determined by pulse amplitude modulation fluorometry. FEMS Microbiol Ecol 59:265–273
Takeuchi N (2001) The altitudinal distribution of snow algae on an Alaska glacier (Gulkana Glacier in the Alaska Range). Hydrol Process 15:3447–3459
Takeuchi N, Kohshima S (2004) A snow algal community on Tyndall Glacier in the Southern Patagonia Icefield, Chile. Arct Antarct Alp Res 36:92–99
Uetake J, Naganuma T, Hebsgaard MB, Kanda H, Kohshima S (2010) Communities of algae and cyanobacteria on glaciers in west Greenland. Polar Sci 4:71–80
Wodniok S, Brinkmann H, Glöckner G, Heidel AJ, Philippe H, Melkonian M, Becker B (2011) Origin of land plants: do conjugating green algae hold the key? BMC Evol Biol 11:104–113
Yoshimura Y, Kohshima S, Ohtani S (1997) A community of snow algae on a Himalayan glacier: change of algal biomass and community structure with altitude. Arct Antarct Alp Res 29:126–137
Acknowledgments
The authors thank Dr. Birgit Sattler, Institute of Ecology, University of Innsbruck, for collecting ice samples at Midtre Lovenbreen in 2007. This study has been supported by the Austrian Science Fund (FWF, P20810 to C.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remias, D., Holzinger, A., Aigner, S. et al. Ecophysiology and ultrastructure of Ancylonema nordenskiöldii (Zygnematales, Streptophyta), causing brown ice on glaciers in Svalbard (high arctic). Polar Biol 35, 899–908 (2012). https://doi.org/10.1007/s00300-011-1135-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1135-6