Abstract
Following a worldwide trend, Brazil is regulating control actions and traceability of medicines Through Board Resolution (TBR) n\({^\mathrm{o}}\). 54, which provides for the establishment of the national system drug control and the mechanisms and precedents for your tracking in the chain of pharmaceuticals. Depending on the responsibility to ensure, ensure the maintenance, enhance quality, promote the safety and efficacy of products to the final consumer and seeking to avoid the risks and adverse health effects, companies, factures or importers, have a responsibility to adopt mechanisms and procedures that address is resolution. This article presents an overview of the needs of serialization and traceability pharma-protection products, as well as value creation opportunities in the implementation of systems and their adequacy to rules for companies that make up the cahate pharmaceutical value.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The improvement program, of the regulatory process of The Brazillian Health Regulatory Agency (Anvisa), established by Decree n\({^\mathrm{o}}\). 422 of April 16, 2008, and also the provisions of Law n\({^\mathrm{o}}\). 11,903, of January 14, 2009, it provides for the implementation of the national system of drug control and the mechanisms and procedures for tracking drugs in the chain of pharmaceutical products; practice that determines the new guidelines and unique identification rules for drug being called UIR.
This resolution is based on the concept of serialization and traceability, Despite widely publicized by the media Brazilian society did not absorb all the concepts involved. Some questions have not been September permanently. When measured technological solutions to a subject, there are several options in both the national and international market, however, there is great difficulty in understanding the new resolution in terms of understanding the standard, as well as the concepts of serialization and traceability which makes complex close the process to implement the solution throughout the chain so that the solution is unique and the data can bring all necessary information at all stages of the selection of raw materials, production, marketing to the end consumer.
This work seeks to demonstrate macro form the concept, methodology, and application with the technological solutions that have been used in Brazil. It is noteworthy that this study has as main objective the drug developers.
2 Theoretical Background
2.1 Information Technology
To Santos [1] using information technology when it comes to health is fundamental prioritize therefore can often be a fine line between over-experience of fatality. In the Industry of Health, there is always an attempt to cut public spending and demand greater efficiency in this industry, several companies associated with the health as the American Hospital Association, the Health Industry Business Communication Council, the National Wholesale Druggists Association, among others, invest in research called Efficient Healthcare Consumer Response. In this research, a strong recommendation is to implement information systems to automate the current processes of the supply chain [2].
The best way to promote the performance of the supply chain, while in parallel, control is the short-term costs, not to colortar budgets, expenses, but invest in process improvements, and technological resources, which will eliminate costs of supply chain [2].
To Taraboulsi [3] it is essential that health institutions have as a tool management systems provided by IT, because that enhance the processes of treatment, spread and transfer of information, add value to serve vices and make decision-making more agile, effective and consistent.
Holland and Nimno [4] report that hospital pharmacies were incorporating advanced technological resources with computerized prescription, automation system for distribution of drugs and computerization processes.
According to Gomes and Reis [5], computer automation as acts as an important tool for rationalization of use of time and agility of activities in the pharmacy.
2.2 Tecnologies Used for Product Traceabilitys
Bar codes to a use widely disseminated. Used in various industries was initially used in the retail market and therefore, widely disseminated in markets. Barcodes have a lot of information and graphs numeric and alphanumeric data. This encoding has intervals of diffuse reflectivity alternating, are represented by rectangles, however, no information, but certainly make typing easier [6]. The GS1 System is based on bar codes and is the global standard used, presenting the data layout to be followed:
-
EAN/UPC Universal Product Code, that is widely used in point of sale (POS) and have an agility in capturing information;
-
DataBar: This code family can be used in POS (point of sale), but has a smaller size and has the capacity to have more information than EAN/UPC.
-
Code 128: Standard Code of GS1 keys. This pattern should not be used for identification at the POS;
-
ITF-14 (Interleaved Two of Five): This standard should not be used for identification at the POS; It can be printed on cardboard;
-
Data Matrix: It has a higher storage capacity being a symbol it with greater capacity than that linear codes.
Table 1 shows a comparison of the technologies used for product traceability, which highlights its main use factors. Through the table is possible to verify that the Radio-Frequency Identification Technology - RFID, the principle is the most suitable for traceability because medicines, among other factors, the minimum data manipulation, which gives security to the supervisory body and its wide range in the distance data capture, which increases safety and mapping process, being able to control not only their location in real time, but act quickly if the exit load predefined script and can avoid the theft of the same [7].
3 Understand Serialization and Traceability of Medicines
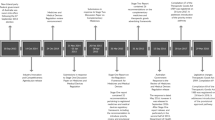
We can divide the process of serialization and traceability in two stages that are serialization and aggregation. In designing serialization, it happens in the coding phase, because at this time are entered the data that references all information as batch identification, expiration dates and the dates and the details of the label and the definition of data [8]. In other words, the serialization is the definition and implementation of a unique identifier, such as a serial number that identifies the individual packages of medicines, your application is through the code printing (2D) and production batch data.
It is important to keep the information in unit doses or rather the lower marketed dose. For this and crucial that printing equipment can print the information in the Data Matrix format, this standard is defined and regulated by ANVISA, such code have the serial number, product registration number, batch, manufacturing and expiry dates that have quality standard [8].
In order to check the printed information, see the example in Fig. 1, the serialization equipment needs of visual inspection systems as well as waste efficient and analyzing the printed data evaluating and making sure they are within the minimum criteria set out in the DRC Standard n\({^\mathrm{o}}\). 54 2013 in order to be accepted. The requirements are [8]:
-
Standard print size.
-
Comparison and analysis of the data reported by the ERP system with the label.
-
Quality Printing.
-
Process analysis and in case of failure in print or evidence of divergence in the data printed a reject process should be established. Standard print size.
3.1 Aggregation
When measured aggregation of drugs, it occurs after the codification of the serialization information, medications that have been identified in photographic form containing the data of aggregates cartridges rows and the data to be easily identifiable to get information regarding the composition, origin and storage. It is important that a label is printed after closing the box when it is complete. The box must have a label that will be unit must be aggregated into a single shipment box so to have a single record doing the same family or group [8].
The shipping box should be inspected and it is important to have the printed record by the system and will have the information of the whole makeup box, and constitute a new code. This new label “father” must be fixed in the shipment box to identify the “children” [8] (Fig. 2).

(Source: [8])
Label aggregation
The TBR n\({^\mathrm{o}}\). 54 of 2013 does not have a mandatory requirement regarding the aggregation of shipping boxes on the pallet, however, to be effective control and traceability during all stages of the process is required data to be stored and sent to the server traceability of the manufacturer and stored in a database for later to take effect the transmission of information to ANVISA where there will also be storage in a database.
For this to happen in a way that facilitates the control of the physical location is important that shipping boxes have an identification number and can easily get the information inspected, and that the place of storage, to be easily identified. So that the process can be completed, after collecting boarding box data and validation of them will require a new label is printed with all units of parents and children and this information should be in a visible place, after “stretching” or “strecht wrap” (placement strectch film) to protect the packing boxes to be sent to the logistics operator.
3.2 Definition of the Production Line
In the consignment of production data step is defined to be included in Datamatrix, such as the data of separation of raw material, weighing the production of semifinished data, reconciliation, release, among other information related to production.
For this phase of the process it is necessary to define a unique serial number for each product unit and for this serial also used the registry number of the product at ANVISA, including batch number, expiry and manufacturing dates (Fig. 3).
4 Methodology
This paper can be considered an exploratory study to generate a structure that will subsequently be tested in future studies, through the development of hypotheses to be investigated; descriptive, by engaging the attempt to control and manipulation of variables; case study, to be returned to the depth, targeting a full contextual analysis of a few facts; and laboratory research, because it was not applied yet [9].
Initially, there was a survey of macro use of technology in the drug supply chain, using materials such as articles published in scientific journals and international very important events. Then we made a study of the methods and drug traceability technologies used in Brazil and the world, and its effectiveness in relation to the following requirements: guarantee information along the supply chain, decrease in counterfeit drugs, traceability lot quickly, with quality that reaches the end customer and information available on the medicine for it.
Based on this knowledge, it concluded the elaboration of cover the reader ways to mitigate serialization and traceability, through a study on strategies for system implementation and adaptation to the TBR n\({^\mathrm{o}}\). 54, 2013. An attempt to improve the existing process and identify future research possibilities.
5 Results and Discussion
The new tracking law until the end of 2016 the entire pharmaceutical industry should follow the new rules. The proposal is that the production stage to consumption registration is made, storage and transmission of data. To provide greater security is being adopted this global trend.
Clearly there will be the expansion of the security when the traceability process is being used for medicinal products. This is a good practice and is also a trend in other countries that are seeking to increase the safety of medicinal products. Avoiding not only fraud, robbery of cargo it, but also providing greater security and protection to the consumer.
In the productive sector, it is the measure will allow the greatest control of all costs of the stages of production and logistics. The process and all the production and distribution chain links are essential for any drug traceability process to work. The links found pharmacies are one of the distribution links that need to inform the reception of this, during the delivery, the submission processes and anomalous situations, such as loss and falsification, the manufacturer of the drug, and the previous link in the supply chain. All existing connection in the chain has responsibility with the pharmaceutical laboratory that all stages of the delivery process until the sale can provide the information to ANVISA the repository of records of the manufacturer’s traceability of events.
To protect consumers may be noted that traceability enables more secure and reliable buyers in the product they are buying. There will also be a feature of industries and government to manage health care costs and be more easily and efficiently, and regulators can monitor more effectively.
6 Conclusions
Traceability of medicines has not been standardized in the world, and that we still have large amounts of counterfeit drugs, which pose a safety hazard to the final consumer, therefore this product without origin does not have the necessary quality.
Traceability of drugs is no longer a competitive factor, but a necessity to get the safety of the product to the end consumer. For the traceability process is accepted, it is necessary that the existing steps are met, the change is planned and the use of appropriate technologies enabling assurance and tracking of the drug to its ultimate destination, and thus its quality, reducing cases of counterfeit medicines and providing a response and faster action, if necessary to withdraw a circulating drug. In that it contributes to the reverse logistics of these products, enabling the sustainability of the process.
References
Santos, G.A.A.: Gestão de Farmácia Hospitalar. Senac, São Paulo (2009)
Ching, H.Y.: Gestão de Estoques na Cadeia de Logística Integrada Supply Chain. Editora Atlas, São Paulo (2007)
Taraboulsi, F.A.: Administração de Hotelaria Hospitalar. Editora Atlas, São Paulo (2009)
Holland, R.W., Nimmo, C.M.: Transitions part 1: beyond pharmaceutical care. Am. J. Health-Syst. Pharm. 56, 1758–1764 (1999)
de Magalhães Gomes, M.J.V., Reis, A.M.M.: Ciências Farmacêuticas: Uma Abordagem em Farmácia Hospitalar. Atheneu, São Paulo (2006)
Swartz, J., Wang, Y.P.: Fundamentals of bar code information theory. Computer 23(4), 74–86 (1990)
Metzner, V.C.V., da Silva, R.F., Cugnasca, C.E.: Modelo de Rastreabilidade de Medicamentos Utilizando Identificação por Radiofrequência, Redes de Sensores Sem Fio e o Conceito de Internet das Coisas. In: XXVIII Congresso Nacional de Pesquisa e Ensino em Transporte, pp. 1–12. ANTP (2014)
Farmaceuticas. http://www.farmaceuticas.com.br
Bowersox, D.J., Closs, D.J., Cooper, M.B.: Supply Chain Logistics Management, vol. 2. McGraw-Hill, New York (2002)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 IFIP International Federation for Information Processing
About this paper
Cite this paper
de Lira Muniz, A.G., Correa, D.A.M., Abe, J.M., do Amaral, F.V., Tomiatti, L.H.C. (2016). Mitigating Serialization and Traceability, a Study on the Strategies for the Implementation of the System and Adaptation to the TBR n\({^\mathrm{o}}.\) 54 2013. In: Nääs, I., et al. Advances in Production Management Systems. Initiatives for a Sustainable World. APMS 2016. IFIP Advances in Information and Communication Technology, vol 488. Springer, Cham. https://doi.org/10.1007/978-3-319-51133-7_53
Download citation
DOI: https://doi.org/10.1007/978-3-319-51133-7_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51132-0
Online ISBN: 978-3-319-51133-7
eBook Packages: Computer ScienceComputer Science (R0)