Abstract
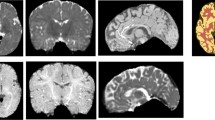
Brains with complex distortion of cerebral anatomy present several challenges to automatic tissue segmentation methods of T1-weighted MR images. First, the very high variability in the morphology of the tissues can be incompatible with the prior knowledge embedded within the algorithms. Second, the availability of MR images of distorted brains is very scarce, so the methods in the literature have not addressed such cases so far. In this work, we present the first evaluation of state-of-the-art automatic tissue segmentation pipelines on T1-weighted images of brains with different severity of congenital or acquired brain distortion. We compare traditional pipelines and a deep learning model, i.e. a 3D U-Net trained on normal-appearing brains. Unsurprisingly, traditional pipelines completely fail to segment the tissues with strong anatomical distortion. Surprisingly, the 3D U-Net provides useful segmentations that can be a valuable starting point for manual refinement by experts/neuroradiologists.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
https://research.cchmc.org/c-mind, NIH contract #s HHSN275200900018C.
- 2.
- 3.
- 4.
References
Ashburner, J., Friston, K.J.: Voxel-based morphometry–the methods. NeuroImage 11(6 Pt 1), 805–821 (2000). https://doi.org/10.1006/nimg.2000.0582
Avants, B., Tustison, N., Wang, D.J.: The Pediatric Template of Brain Perfusion (PTBP) (2015). https://doi.org/10.6084/m9.figshare.923555.v20
Çiçek, Ö., Abdulkadir, A., Lienkamp, S.S., Brox, T., Ronneberger, O.: 3D U-Net: learning dense volumetric segmentation from sparse annotation. In: Ourselin, S., Joskowicz, L., Sabuncu, M.R., Unal, G., Wells, W. (eds.) MICCAI 2016. LNCS, vol. 9901, pp. 424–432. Springer, Cham (2016). https://doi.org/10.1007/978-3-319-46723-8_49
Cullen, N.C., Avants, B.B.: Convolutional neural networks for rapid and simultaneous brain extraction and tissue segmentation. In: Spalletta, G., Piras, F., Gili, T. (eds.) Brain Morphometry. N, vol. 136, pp. 13–34. Springer, New York (2018). https://doi.org/10.1007/978-1-4939-7647-8_2
Dale, A.M., Fischl, B., Sereno, M.I.: Cortical surface-based analysis I. Segmentation and surface reconstruction. NeuroImage 9(2), 179–194 (1999). https://doi.org/10.1006/nimg.1998.0395
Evans, A.C.: The NIH MRI study of normal brain development. NeuroImage 30(1), 184–202 (2006). https://doi.org/10.1016/j.neuroimage.2005.09.068
Jenkinson, M., Beckmann, C.F., Behrens, T.E.J., Woolrich, M.W., Smith, S.M.: FSL. NeuroImage 62(2), 782–790 (2012). https://doi.org/10.1016/j.neuroimage.2011.09.015
Ledig, C., et al.: Robust whole-brain segmentation: application to traumatic brain injury. Med. Image Anal. 21(1), 40–58 (2015). https://doi.org/10.1016/j.media.2014.12.003
Abadi, M., et al.: TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems (2015)
Moeskops, P., Viergever, M.A., Mendrik, A.M., de Vries, L.S., Benders, M.J.N.L., Išgum, I.: Automatic segmentation of MR brain images with a convolutional neural network. IEEE Trans. Med. Imaging 35(5), 1252–1261 (2016). https://doi.org/10.1109/TMI.2016.2548501
Rajchl, M., Pawlowski, N., Rueckert, D., Matthews, P.M., Glocker, B.: NeuroNet: Fast and Robust Reproduction of Multiple Brain Image Segmentation Pipelines (2018)
Ronneberger, O., Fischer, P., Brox, T.: U-Net: convolutional networks for biomedical image segmentation. In: Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F. (eds.) MICCAI 2015. LNCS, vol. 9351, pp. 234–241. Springer, Cham (2015). https://doi.org/10.1007/978-3-319-24574-4_28
Roy, S., et al.: Subject-specific sparse dictionary learning for atlas-based brain MRI segmentation. IEEE J. Biomed. Health Inf. 19(5), 1598–1609 (2015). https://doi.org/10.1109/JBHI.2015.2439242
Shao, M., et al.: Brain ventricle parcellation using a deep neural network: application to patients with ventriculomegaly. NeuroImage Clin. 23, 101871 (2019). https://doi.org/10.1016/j.nicl.2019.101871
Tustison, N.J., et al.: Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage 99, 166–179 (2014). https://doi.org/10.1016/j.neuroimage.2014.05.044
Yogananda, C.G.B., Wagner, B.C., Murugesan, G.K., Madhuranthakam, A., Maldjian, J.A.: A deep learning pipeline for automatic skull stripping and brain segmentation. In: 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), pp. 727–731 (2019). https://doi.org/10.1109/ISBI.2019.8759465, iSSN: 1945-7928
Acknowledgment
Data used in the preparation of this article were obtained from the C-MIND Data Repository created by the C-MIND study of Normal Brain Development. This is a multisite, longitudinal study of typically developing children from ages newborn through young adulthood conducted by Cincinnati Children’s Hospital Medical Center and UCLA and supported by the National Institute of Child Health and Human Development (Contract #s HHSN275200900018C). A listing of the participating sites and a complete listing of the study investigators can be found at https://research.cchmc.org/c-mind. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Amorosino, G. et al. (2020). Automatic Tissue Segmentation with Deep Learning in Patients with Congenital or Acquired Distortion of Brain Anatomy. In: Kia, S.M., et al. Machine Learning in Clinical Neuroimaging and Radiogenomics in Neuro-oncology. MLCN RNO-AI 2020 2020. Lecture Notes in Computer Science(), vol 12449. Springer, Cham. https://doi.org/10.1007/978-3-030-66843-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-66843-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66842-6
Online ISBN: 978-3-030-66843-3
eBook Packages: Computer ScienceComputer Science (R0)