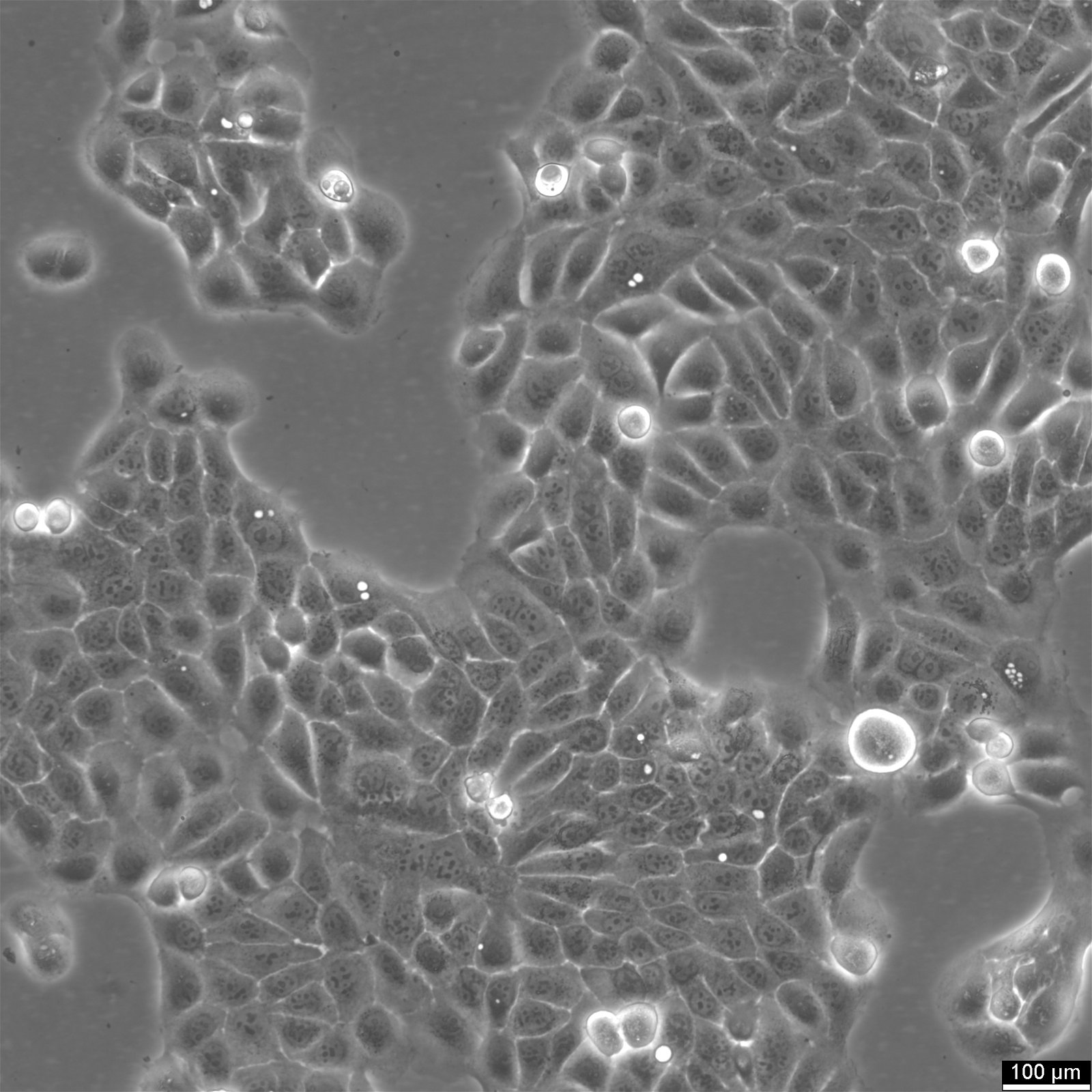
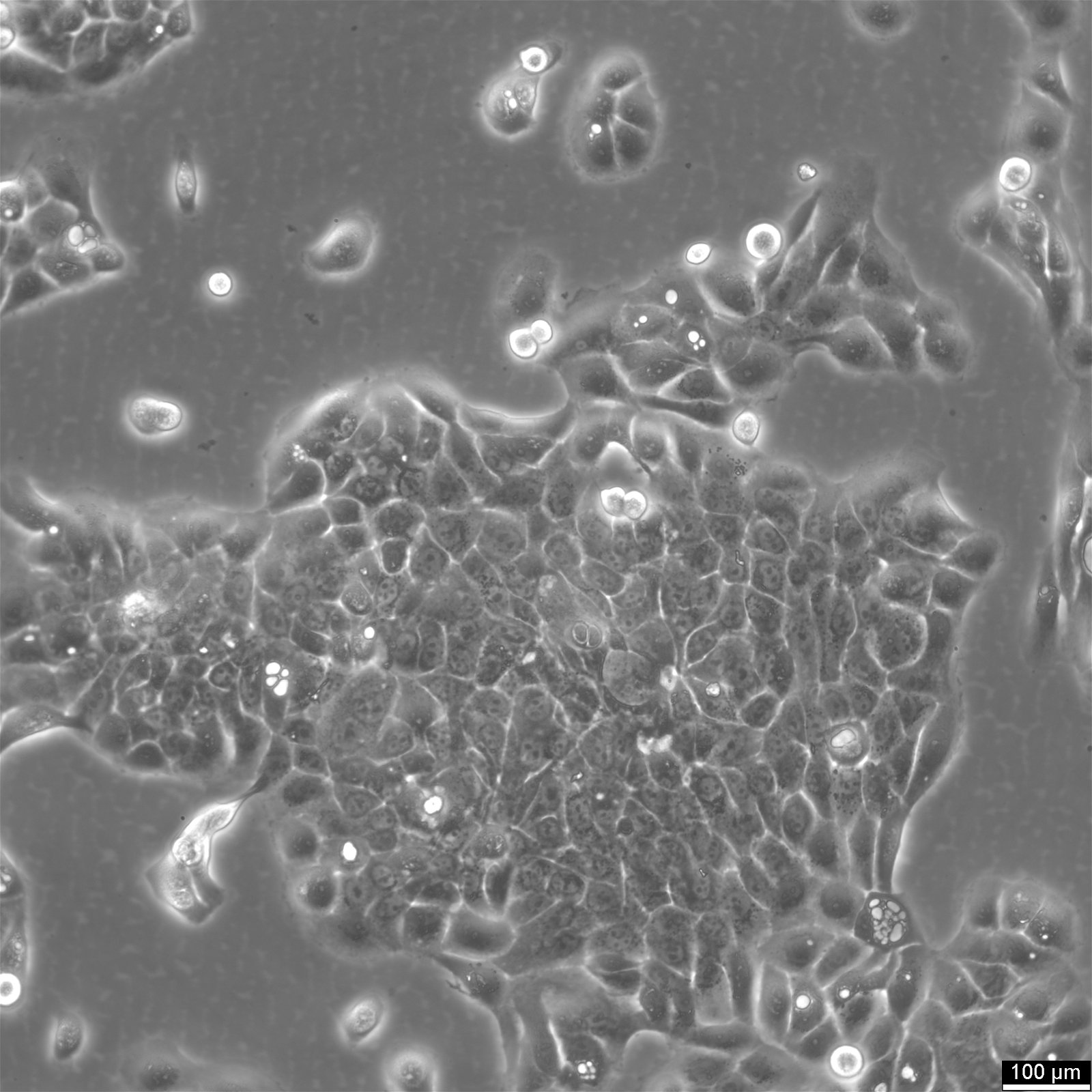
HaCaT Cells









Essential facts about Hacat cells
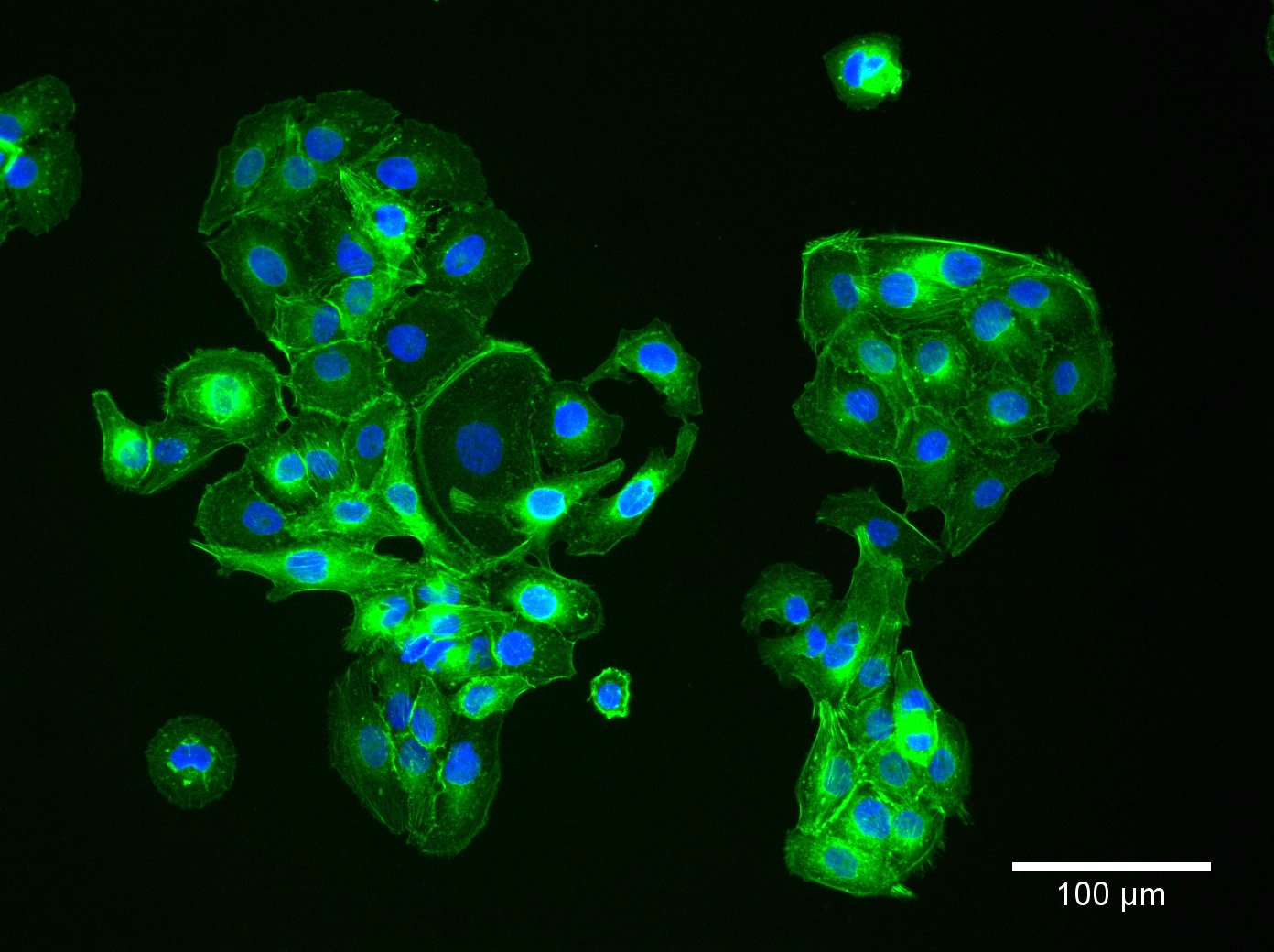
| Description | HaCaT cells are a pivotal model in dermatological research, offering insights into the complex mechanisms of skin biology and pathology. The spontaneously immortalized HaCaT cell line is derived from adult human epidermal cells and retains the capacity to proliferate and undergo differentiation, similar to basal keratinocytes in vivo. HaCaT cells serve as a robust platform for investigating the epidermal differentiation process and studying the epidermal differentiation markers essential for maintaining skin integrity. The susceptibility of HaCaT cells to apoptosis and their sensitivity to apoptosis-inducing agents are extensively studied, particularly in the context of cytotoxic agents like RIPL. Researchers assess these agents' cytotoxicities and the extent of cytotoxicity using HaCaT cells, utilizing techniques such as fluorescence microscopy to visualize cellular changes. Researchers have leveraged HaCaT cells to examine the effects of various agents, including antimicrobial substrates and their influence on cell viability. These cells are an excellent substrate for testing antimicrobial biomaterials and antimicrobial atelocollagen substrates, crucial for skin repair and medical applications. The HaCaT epidermal line also plays a crucial role in studying cellular senescence, cytokines, and gene expression profiles related to aging and chronic diseases. The transcriptional profiles of HaCaT cells, including the role of κB and microRNAs, provide insight into the regulatory mechanisms at the molecular level. The HaCaT keratinocyte line, with their characteristics as epidermal keratinocytes, offers a tractable system for dissecting the intricate interplay between epidermal cells and the immune system, specifically the role of keratinocytes in disease states. They enable the exploration of epigenetic modifications and their influence on the differentiation of keratinocytes, including the formation of the cornified envelope, a key feature in the skin's barrier function. In summary, HaCaT cells are an indispensable model in dermatological research, facilitating a deeper understanding of skin biology and pathology through their resemblance to basal keratinocytes and their ability to undergo cell growth and differentiation. Their application spans from studying epidermal differentiation and antimicrobial effects to exploring cellular responses such as apoptosis, making them a cornerstone in cell biology and biomedical research. |
|---|---|
| Organism | Human |
| Tissue | Skin |
Details
| Age | 62 years |
|---|---|
| Gender | Male |
| Ethnicity | Caucasian |
| Cell type | Keratinocytes with a diameter of 20-25 micrometer. |
| Growth properties | Adherent |
Documentation
| Citation | HaCaT (Cytion catalog number 300493) |
|---|---|
| Biosafety level | 1 |
| Depositor | DKFZ, Heidelberg |
Genetics of the HaCaT keratinocyte cell line
| Tumorigenic | No |
|---|---|
| Karyotype | Aneuploid (hypotetraploid) |
Handling the Hacat cell line
| Culture Medium | DMEM, w: 4.5 g/L Glucose, w: 4 mM L-Glutamine, w: 1.5 g/L NaHCO3, w: 1.0 mM Sodium pyruvate (Cytion article number 820300a) |
|---|---|
| Medium supplements | Supplement the medium with 10% FBS |
| Passaging solution | The 1:1 mixture of EDTA (stock. 0.05%) and trypsin (stock: 0.1%) must be prepared each time ahead of detaching the cells using PBS without Ca2+ and Mg2+ to provide a physiologic osmolarity. Ready-to-use mixtures of trypsin/EDTA are not recommended, as this may result in cell clumps. As an alternative, TrypLE Express (Life Technologies) instead of trypsin/EDTA can be used. The protocol of the manufacturer should be followed. |
| Doubling time | The doubling time of HaCaT cells is 28 hours. |
| Subculturing |
|
| Split ratio | A ratio of 1:5 to 1:10 is recommended |
| Seeding density | 1 x 10^4 cells/cm^2 |
| Fluid renewal | 2 times per week |
| Freeze medium | CM-1 (Cytion catalog number 800100) or CM-ACF (Cytion catalog number 806100) |
| Handling of cryopreserved cultures |
|
Quality assurance
| Sterility | Mycoplasma contamination is excluded using both PCR-based assays and luminescence-based mycoplasma detection methods. To ensure there is no bacterial, fungal, or yeast contamination, cell cultures are subjected to daily visual inspections. |
|---|---|
| STR profile |
Amelogenin: x,x
CSF1PO: 9,11
D13S317: 10,12
D16S539: 9,12
D5S818: 12
D7S820: 9,11
TH01: 09. Mrz
TPOX: 11,12
vWA: 16,17
D3S1358: 16
D21S11: 28,30.2
D18S51: 12
Penta E: 7,12
Penta D: 11,13
D8S1179: 14
FGA: 24
D1S1656: 11,12
D2S1338: 17,25
D12S391: 18,23
D19S433: 13,14
|
| HLA alleles |
A*: 01.01.1900 07:01
B*: 01.01.1900 16:01, 02.01.1900 03:01
C*: 03:04:01, 15:02:01
DRB1*: 04:01:01, 15:01:01
DQA1*: 01:02:01, 03:03:01
DQB1*: 03:01:01, 06:02:01
DPB1*: 03:01:01, 04:01:01
E: 01:03:01, 01:03:02
|
Required Products
What sets DMEM apart from other media is its unique composition. It contains an impressive fourfold increase in amino acid and vitamin concentration compared to the original Eagle's Minimal Essential Medium. Initially developed with low glucose (1 g/L) and sodium pyruvate, DMEM is frequently employed with higher glucose levels, either with or without sodium pyruvate. Notably, DMEM does not contain proteins, lipids, or growth factors, necessitating supplementation. To achieve optimal growth, a common approach is to supplement DMEM with 10% Fetal Bovine Serum (FBS). Additionally, DMEM employs a sodium bicarbonate buffer system (3.7 g/L), requiring a 5-10% CO2 environment to maintain a physiological pH.
Dulbecco's Modified Eagle Medium (DMEM) is highly regarded among defined media for cell and tissue culture, catering to the growth needs of various adherent cell phenotypes. It surpasses the original Eagle's Medium, developed in the 1950s for cultivating chicken cells, through the enhanced supplementary formulation known as Dulbecco's modification. This modification significantly elevates the content of select amino acids and vitamins up to fourfold compared to the original medium.
In the field of cell culture, DMEM plays a vital role as a growth medium for different cell types, including primary cells, stem cells, and transformed cells. Researchers also employ the modified version of DMEM for a wide array of research applications, such as drug discovery, tissue engineering, and the study of cell signaling pathways.
Quality control
pH = 7.2 +/
- 0.02 at 20-25°C.
Each lot has been tested for sterility and absence of mycoplasma and bacteria.
Maintenance
Keep refrigerated at +2°C to +8°C in the dark. Freezing and warming up to +37° C minimize the quality of the product.
Do not heat the medium to more than 37° C or use uncontrollable sources of heat (e.g., microwave appliances).
If only a part of the medium is to be used, remove this amount from the bottle and warm it up at room temperature.
Shelf life for any medium except for the basic medium is 8 weeks from the date of manufacture.
Composition
Components
mg/L
Inorganic Salts
Calcium chloride anhydrous
200,00
Iron (III) nitrate x 9H2O
0,10
Magnesium sulfate anhydrous
97,66
Potassium chloride
400,00
Sodium chloride
6.400,00
Sodium dihydrogen phosphateanhydrous
108,69
Other Components
D(+)-Glucose anhydrous
4.500,00
Sodium pyruvate
110,00
Phenol red
15,00
NaHCO3
1.500,00
Amino acids
L-Arginine x HCl
84,00
L-Cystine x 2HCl
62,58
L-Glutamine
584,00
Glycine
30,00
L-Histidine x HCl x H2O
42,00
L-Isoleucine
104,80
L-Leucine
104,80
L-Lysine x HCl
146,20
L-Methionine
30,00
L-Phenylalanine
66,00
L-Serine
42,00
L-Threonine
95,20
L-Tryptophan
16,00
L-Tyrosine x Na
103,79
L-Valine
93,60
Vitamins
D-Calcium pantothenate
4,00
Choline chloride
4,00
Folic acid
4,00
myo-Inositol
7,00
Nicotinamide
4,00
Pyridoxine x HCl
4,00
Riboflavin
0,40
Thiamine x HCl
4,00
Key features of Freeze Medium CM-1 include:
Broad Compatibility: Effective for a wide range of cell types, including primary cells, stem cells, and established cell lines.
High Viability: Optimized to maximize post-thaw cell recovery and viability, ensuring reliable experimental outcomes.
Ready-to-Use: Conveniently prepared and sterilized for immediate application, reducing preparation time and risk of contamination.
Enhanced Stability: Maintains consistent performance under standard cryopreservation conditions, ensuring reproducible results.
Long Shelf Life: CM-1 is a serum-containing, ready-to-use cryopreservation medium that can be stored in the refrigerator for up to one year.
Using CM-1 for Freezing Cells
To use CM-1 for freezing both adherent and suspension cells, follow these steps:
For adherent cells, wash and dissociate them from the culture substrate. For suspension cells, proceed directly to the next step.
Count the cells to ensure they are at the proper concentration.
Centrifuge the cells to pellet them, then resuspend in CM-1 freeze medium.
Transfer the resuspended cells into cryovials.
Use a slow-freezing method before transferring the cells to long-term storage.
🥶 Method
🔍 Description
💡 Steps
❄️
Manual Freezing
A step-by-step method involving gradual temperature reduction to ensure cell viability.
1️⃣ Place cells in freeze medium in a 4°C freezer for 40 minutes.
2️⃣ Transfer to a -80°C freezer for 24 hours.
3️⃣ Store cells in liquid nitrogen for long-term preservation.
🧊
Using Mr. Frosty
A convenient device that allows for controlled freezing rates without electrical power.
1️⃣ Prepare cells in cryovials with freeze medium.
2️⃣ Place cryovials in Mr. Frosty container.
3️⃣ Store at -80°C for 24 hours before transferring to liquid nitrogen.
🧬
Controlled-Rate Freezer
A high-precision freezer by Thermo Fisher or other manufacturers designed for controlled temperature reduction.
1️⃣ Program the device to gradually decrease the temperature.
2️⃣ Place prepared cells in the freezer.
3️⃣ After the freezing cycle, transfer cells to liquid nitrogen.
Store the cryovials at temperatures below -130°C or in liquid nitrogen for long-term preservation.
Ingredients
Contains FBS, DMSO, Glucose, Salts
Buffering capacity: pH = 7.2 to 7.6
Cytion’s Freeze Medium CM-1 offers a reliable solution for cryopreservation, ensuring high cell viability and functionality post-thaw for a wide range of research applications.
- A Gentle Alternative to Trypsin
Accutase is a cell detachment solution that is revolutionizing the cell culture industry. It is a mix of proteolytic and collagenolytic enzymes that mimics the action of trypsin and collagenase. Unlike trypsin, Accutase does not contain any mammalian or bacterial components and is much gentler on cells, making it an ideal solution for the routine detachment of cells from standard tissue culture plasticware and adhesion coated plasticware. In this blog post, we will explore the benefits and uses of Accutase and how it is changing the game in cell culture.
Advantages of Accutase
Accutase has several advantages over traditional trypsin solutions. Firstly, it can be used whenever gentle and efficient detachment of any adherent cell line is needed, making it a direct replacement for trypsin. Secondly, Accutase works extremely well on embryonic and neuronal stem cells, and it has been shown to maintain the viability of these cells after passaging. Thirdly, Accutase preserves most epitopes for subsequent flow cytometry analysis, making it ideal for cell surface marker analysis.
Additionally, Accutase does not need to be neutralized when passaging adherent cells. The addition of more media after the cells are split dilutes Accutase so it is no longer able to detach cells. This eliminates the need for an inactivation step and saves time for cell culture technicians. Finally, Accutase does not need to be aliquoted, and a bottle is stable in the refrigerator for 2 months.
Applications of Accutase
Accutase is a direct replacement for trypsin solution and can be used for the passaging of cell lines. Additionally, Accutase performs well when detaching cells for the analysis of many cell surface markers using flow cytometry and for cell sorting. Other downstream applications of Accutase treatment include analysis of cell surface markers, virus growth assay, cell proliferation, tumor cell migration assays, routine cell passage, production scale-up (bioreactor), and flow cytometry.
Composition of Accutase
Accutase contains no mammalian or bacterial components and is a natural enzyme mixture with proteolytic and collagenolytic enzyme activity. It is formulated at a much lower concentration than trypsin and collagenase, making it less toxic and gentler, but just as effective.
Efficiency of Accutase
Accutase has been shown to be efficient in detaching primary and stem cells and maintaining high cell viability compared to animal origin enzymes such as trypsin. 100% of cells are recovered after 10 minutes, and there is no harm in leaving cells in Accutase for up to 45 minutes, thanks to autodigestion of Accutase.
In summary
In conclusion, Accutase is a powerful solution that is changing the game in cell culture. With its gentle nature, efficiency, and versatility, Accutase is the ideal alternative to trypsin. If you are looking for a reliable and efficient solution for cell detachment, Accutase is the solution for you.
Phosphate-buffered saline (PBS) is a versatile buffer solution used in many biological and chemical applications, as well as tissue processing. Our PBS solution is formulated with high-quality ingredients to ensure a constant pH during experiments. The osmolarity and ion concentrations of our PBS solution are matched to those of the human body, making it isotonic and non-toxic to most cells.
Composition of our PBS Solution
Our PBS solution is a pH-adjusted blend of ultrapure-grade phosphate buffers and saline solutions. At a 1X working concentration, it contains 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4. We have chosen this composition based on CSHL protocols and Molecular cloning by Sambrook, which are well-established standards in the research community.
Applications of our PBS Solution
Our PBS solution is ideal for a wide range of applications in biological research. Its isotonic and non-toxic properties make it perfect for substance dilution and cell container rinsing. Our PBS solution with EDTA can also be used to disengage attached and clumped cells. However, it is important to note that divalent metals such as zinc cannot be added to PBS as this may result in precipitation. In such cases, Good's buffers are recommended. Moreover, our PBS solution has been shown to be an acceptable alternative to viral transport medium for the transport and storage of RNA viruses, such as SARS-CoV-2.
Storage of our PBS Solution
Our PBS solution can be stored at room temperature, making it easy to use and access.
To sum up
In summary, our PBS solution is an essential component in many biological and chemical experiments. Its isotonic and non-toxic properties make it suitable for numerous applications, from cell culture to viral transport medium. By choosing our high-quality PBS solution, researchers can optimize their experiments and ensure accurate and reliable results.
Composition
Components
mg/L
Inorganic Salts
Potassium chloride
200,00
Potassium dihydrogen phosphate
200,00
Sodium chloride
8,000.00
di-Sodium hydrogen phosphate anhydrous
1,150.00