Nitrile-containing pharmaceuticals: target, mechanism of action, and their SAR studies
- PMID: 34778767
- PMCID: PMC8528211
- DOI: 10.1039/d1md00131k
Nitrile-containing pharmaceuticals: target, mechanism of action, and their SAR studies
Abstract
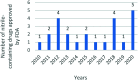
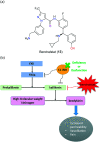
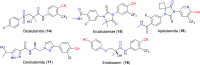
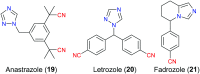
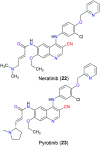
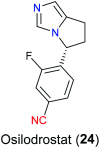
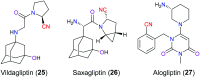
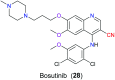
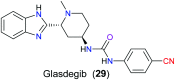
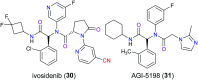
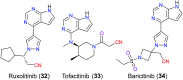
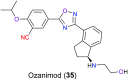
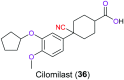
The nitrile group is an important functional group widely found in both pharmaceutical agents and natural products. More than 30 nitrile-containing pharmaceuticals have been approved by the FDA for the management of a broad range of clinical conditions in the last few decades. Incorporation of a nitrile group into lead compounds has gradually become a promising strategy in rational drug design as it can bring additional benefits including enhanced binding affinity to the target, improved pharmacokinetic profile of parent drugs, and reduced drug resistance. This paper reviews the existing drugs with a nitrile moiety that have been approved or in clinical trials, involving their targets, molecular mechanism of pharmacology and SAR studies, and classifies them into different categories based on their clinical usages.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

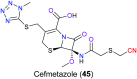
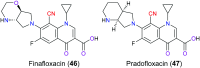
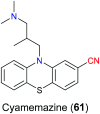
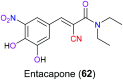
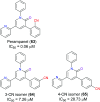
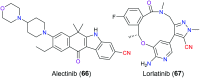
Similar articles
-
Pyrazole-containing pharmaceuticals: target, pharmacological activity, and their SAR studies.RSC Med Chem. 2022 Aug 26;13(11):1300-1321. doi: 10.1039/d2md00206j. eCollection 2022 Nov 16. RSC Med Chem. 2022. PMID: 36439976 Free PMC article. Review.
-
Indole-containing pharmaceuticals: targets, pharmacological activities, and SAR studies.RSC Med Chem. 2024 Jan 30;15(3):788-808. doi: 10.1039/d3md00677h. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516587 Review.
-
Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group.Mar Drugs. 2023 Jan 31;21(2):103. doi: 10.3390/md21020103. Mar Drugs. 2023. PMID: 36827144 Free PMC article.
-
Erlotinib: CP 358774, NSC 718781, OSI 774, R 1415.Drugs R D. 2003;4(4):243-8. doi: 10.2165/00126839-200304040-00006. Drugs R D. 2003. PMID: 12848590
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Review.
Cited by
-
Correlating Predicted Reactivities with Experimental Inhibition Data of Covalent ChlaDUB1 Inhibitors.ACS Med Chem Lett. 2024 Sep 10;15(10):1708-1714. doi: 10.1021/acsmedchemlett.4c00267. eCollection 2024 Oct 10. ACS Med Chem Lett. 2024. PMID: 39411534
-
A single diiron enzyme catalyses the oxidative rearrangement of tryptophan to indole nitrile.Nat Chem. 2024 Sep 16. doi: 10.1038/s41557-024-01603-z. Online ahead of print. Nat Chem. 2024. PMID: 39285206
-
Critical Evaluation of Polarizable and Nonpolarizable Force Fields for Proteins Using Experimentally Derived Nitrile Electric Fields.J Am Chem Soc. 2024 Mar 13;146(10):6983-6991. doi: 10.1021/jacs.3c14775. Epub 2024 Feb 28. J Am Chem Soc. 2024. PMID: 38415598 Free PMC article.
-
Mechanistic Investigation of the Nickel-Catalyzed Transfer Hydrocyanation of Alkynes.ACS Catal. 2023 Aug 16;13(17):11548-11555. doi: 10.1021/acscatal.3c02977. eCollection 2023 Sep 1. ACS Catal. 2023. PMID: 37671177 Free PMC article.
-
Covalent-reversible peptide-based protease inhibitors. Design, synthesis, and clinical success stories.Amino Acids. 2023 Dec;55(12):1775-1800. doi: 10.1007/s00726-023-03286-1. Epub 2023 Jun 17. Amino Acids. 2023. PMID: 37330416 Review.
References
-
- Fleming F. F. Nat. Prod. Rep. 1999;16:597–606. doi: 10.1039/A804370A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous


